Beyond the Bag: A Comprehensive Guide to the Blood Transfusion Set
Jul 22,2025
I. Introduction to Blood Transfusion Sets
Blood transfusion is a life-saving medical procedure that involves transferring blood or blood components from a donor into a recipient's bloodstream.
The journey of blood transfusion, and consequently the evolution of the devices used for it, spans centuries.
In modern medicine, the blood transfusion set is an indispensable tool, playing a vital role in a vast array of clinical scenarios.
II. Components of a Standard Blood Transfusion Set
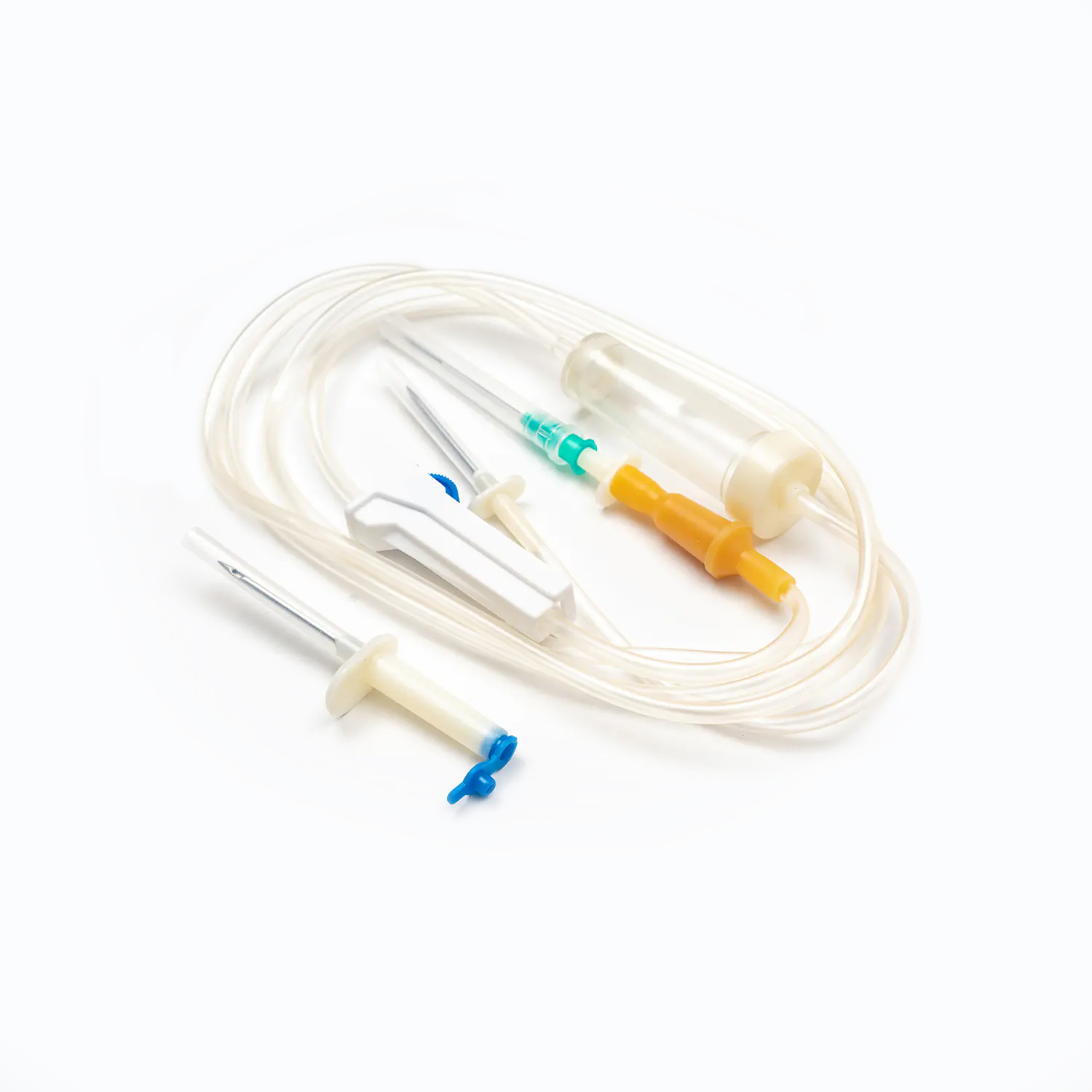
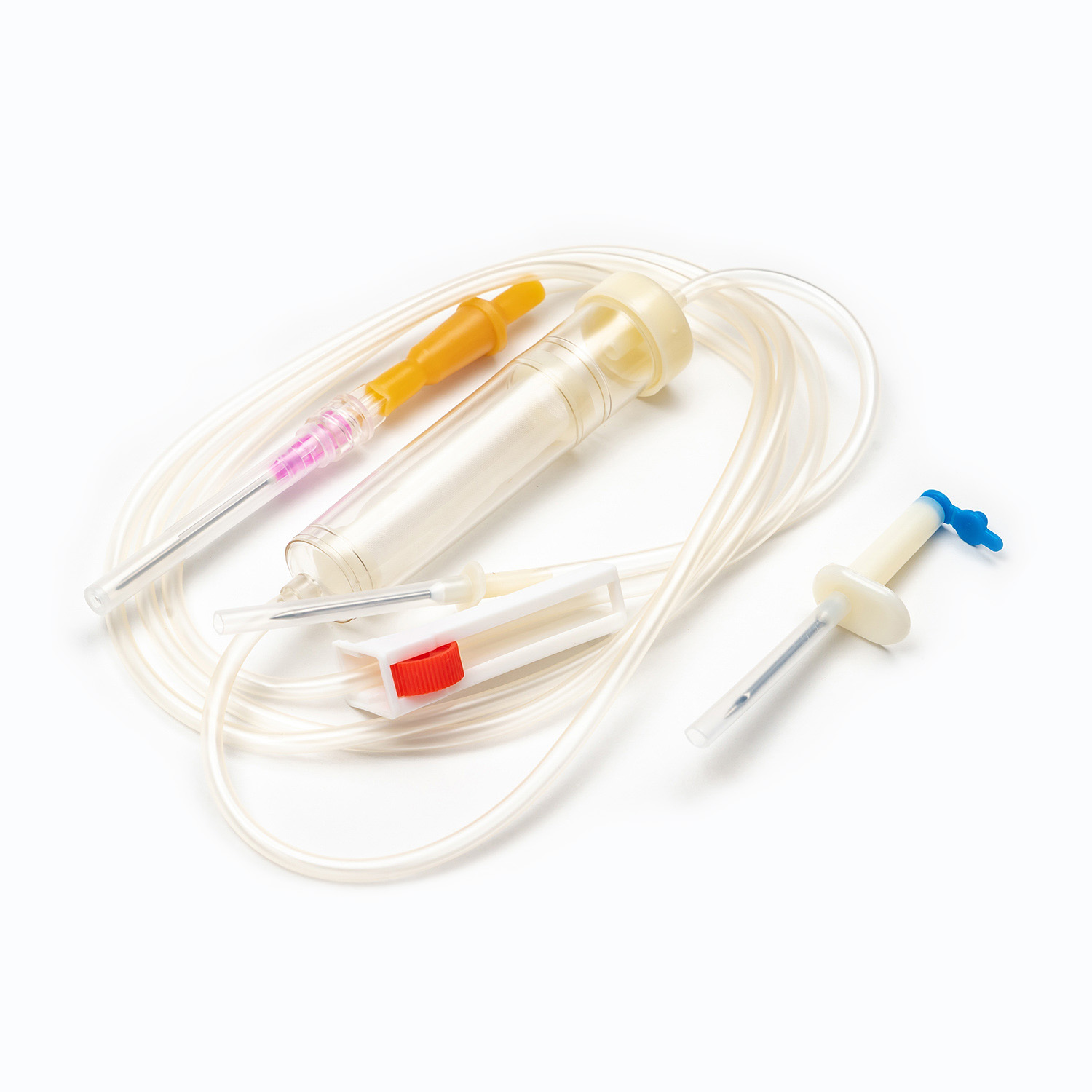
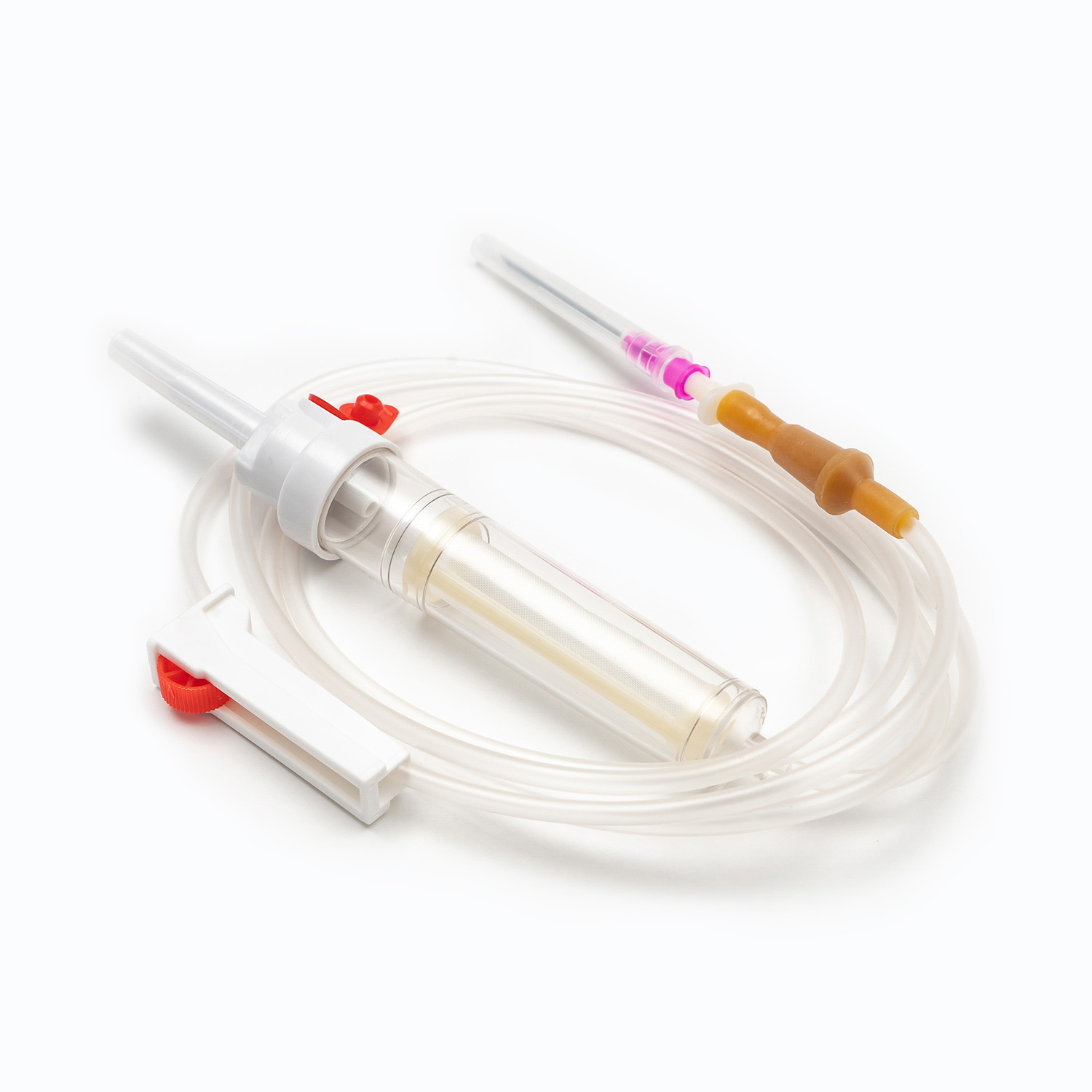
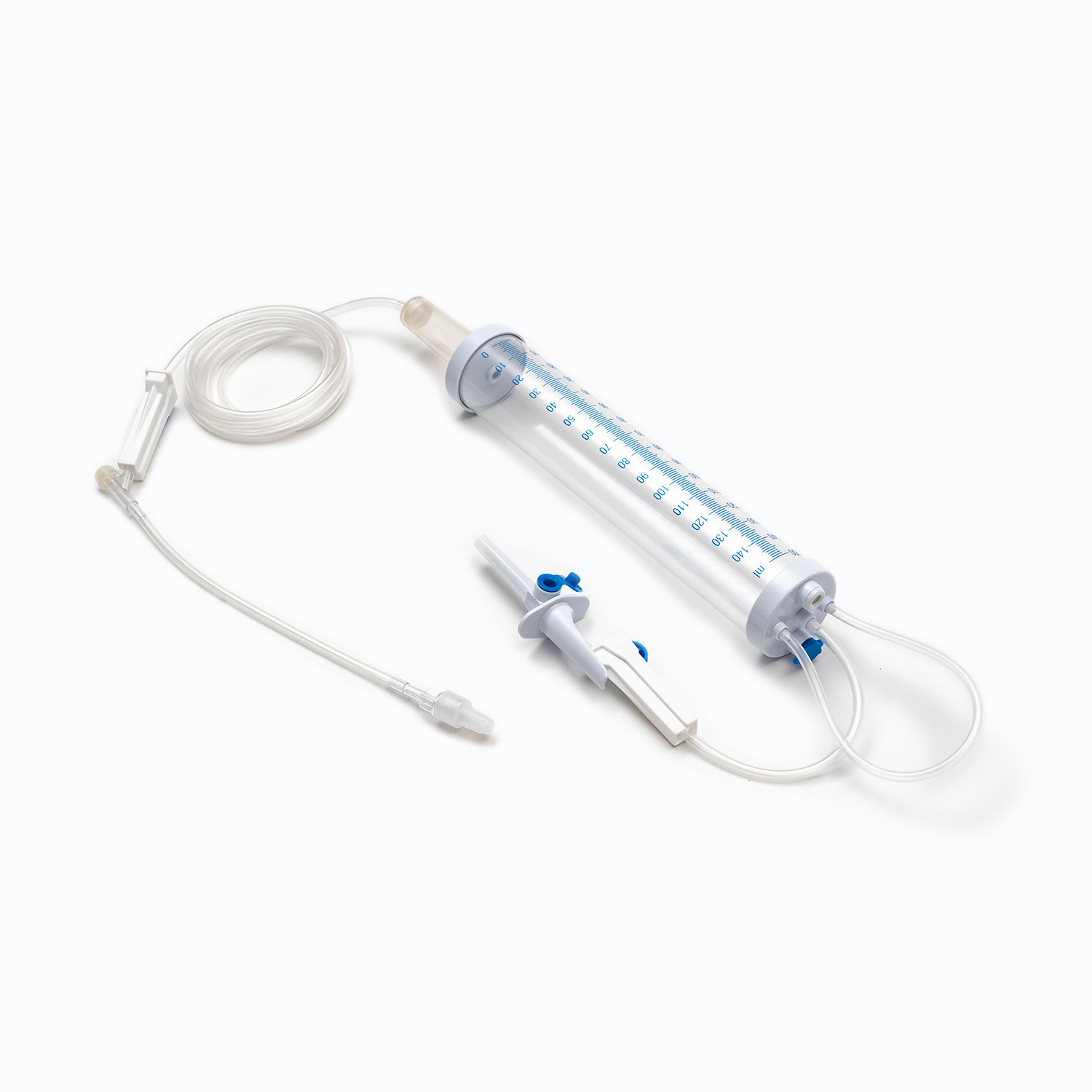
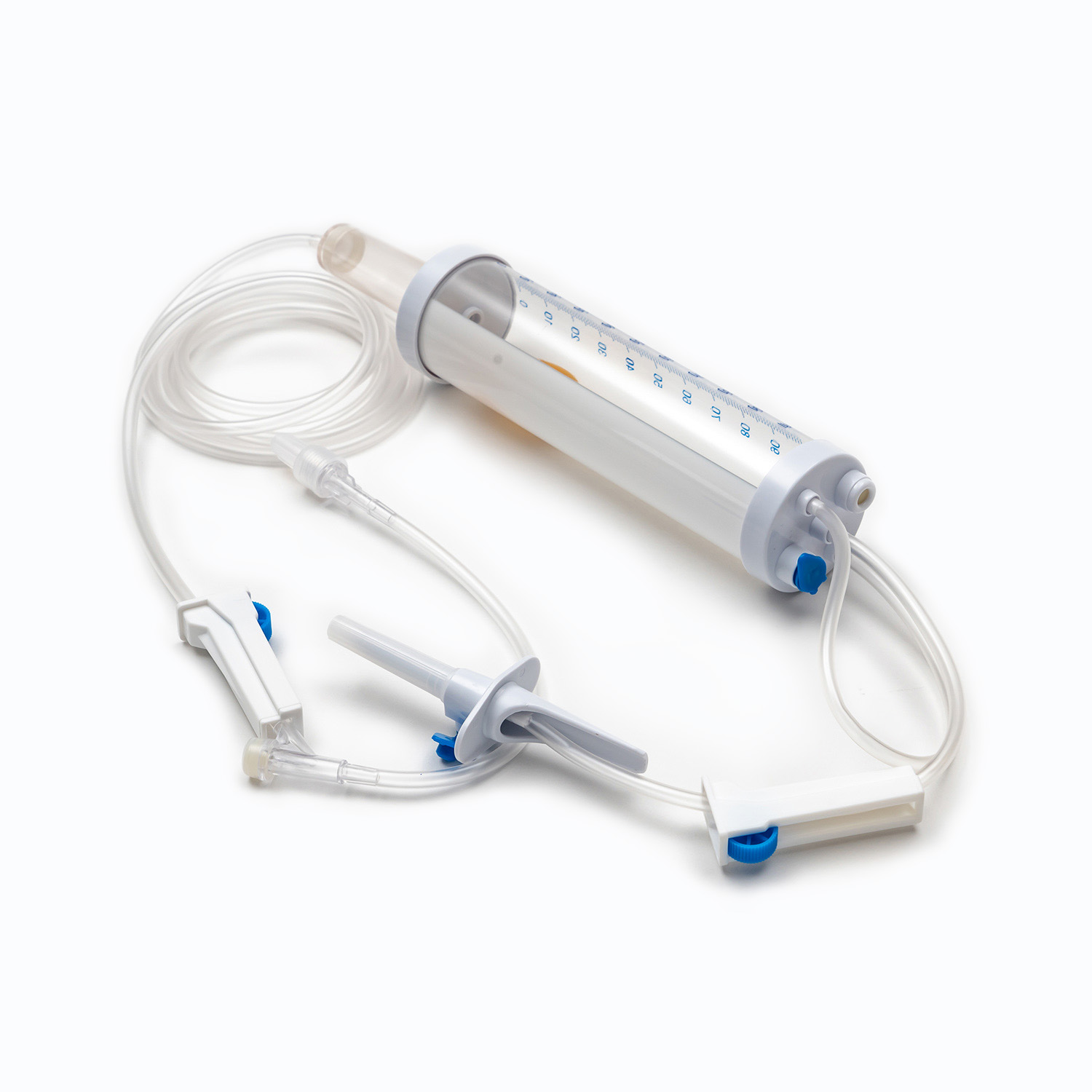
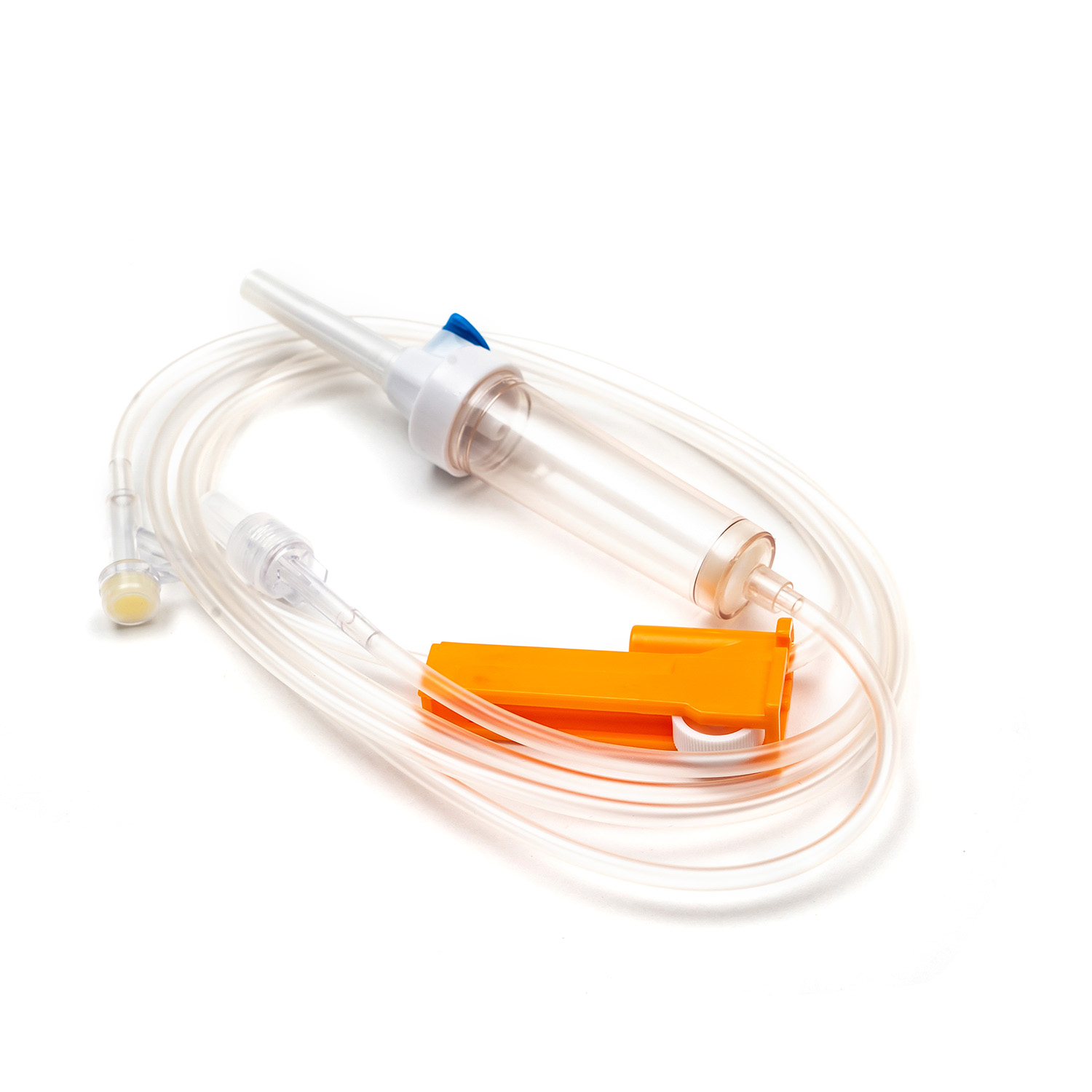
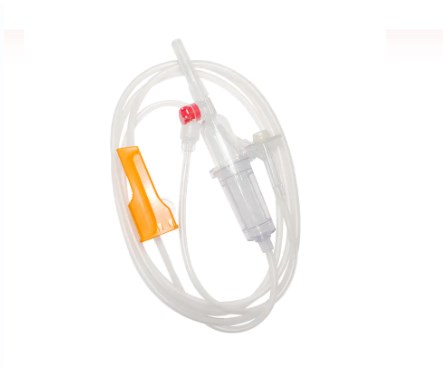
A standard blood transfusion set, though seemingly simple, is a marvel of medical engineering designed for precision and patient safety. Each component plays a vital role in ensuring the effective and sterile delivery of blood products.
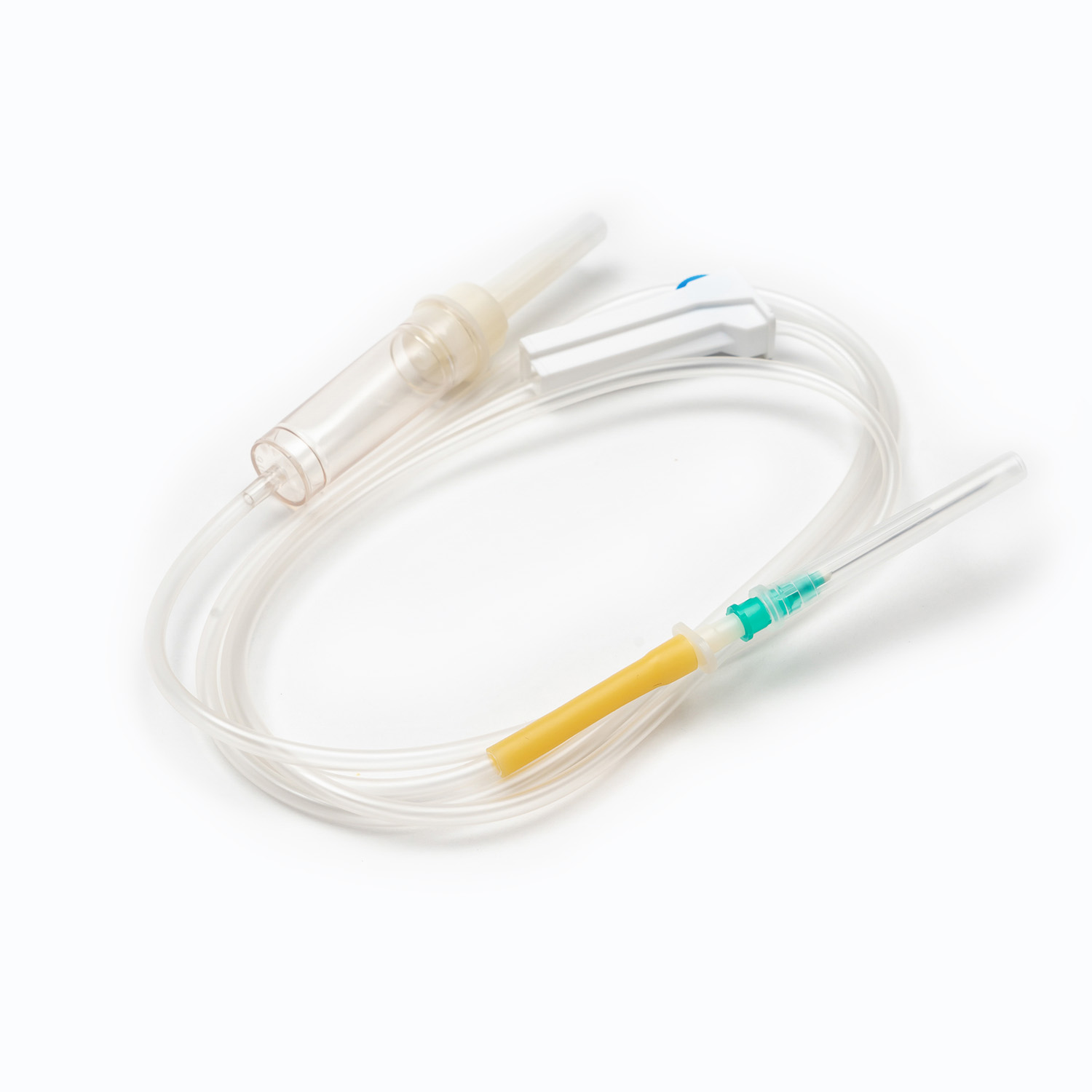
Spike/Piercing Device
The spike, or piercing device, is the rigid, sharp, plastic component at the top of the transfusion set. Its primary function is to securely and aseptically penetrate the port of the blood bag. Designed for a snug fit, it prevents leakage and maintains a closed system, which is crucial for preventing contamination of the blood product.
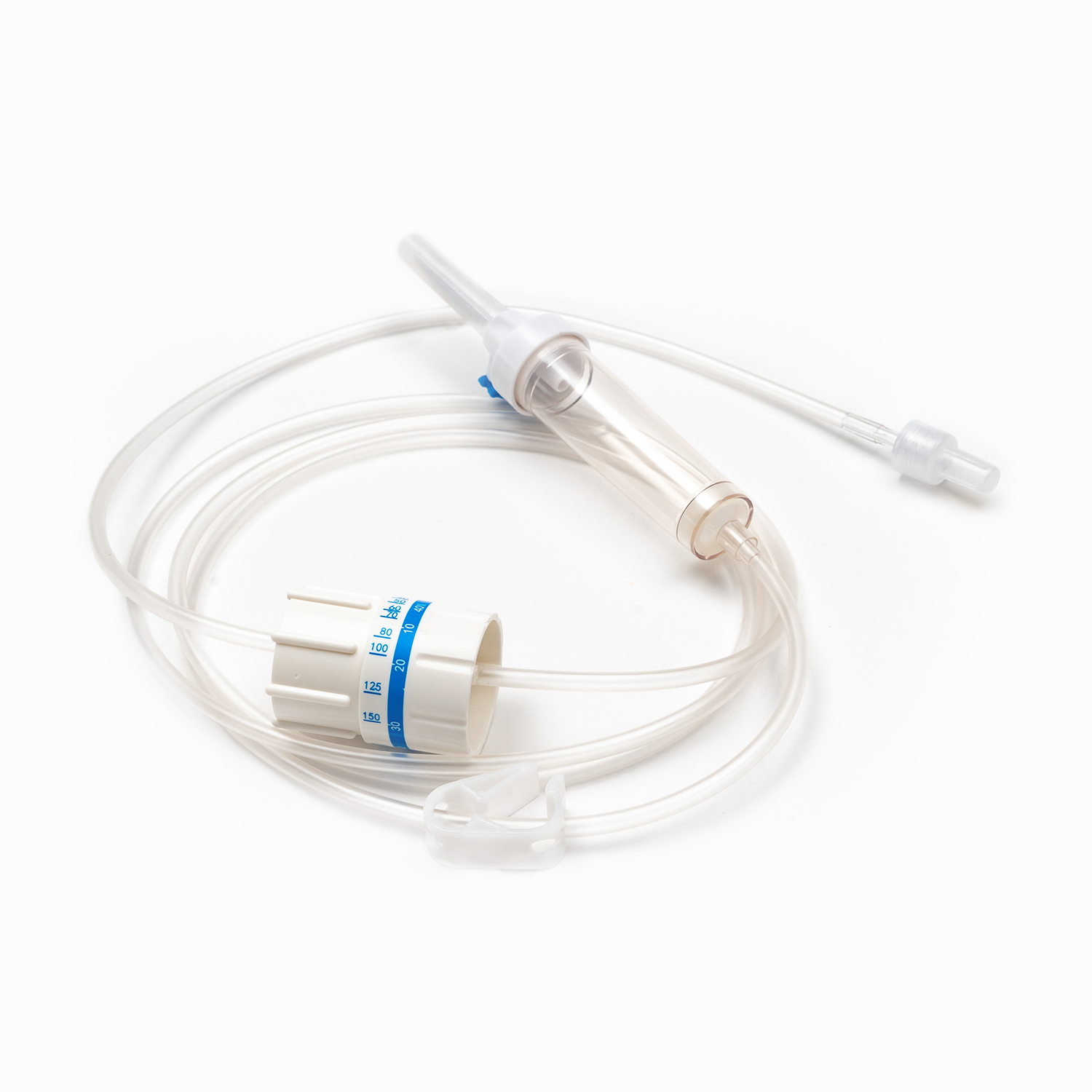
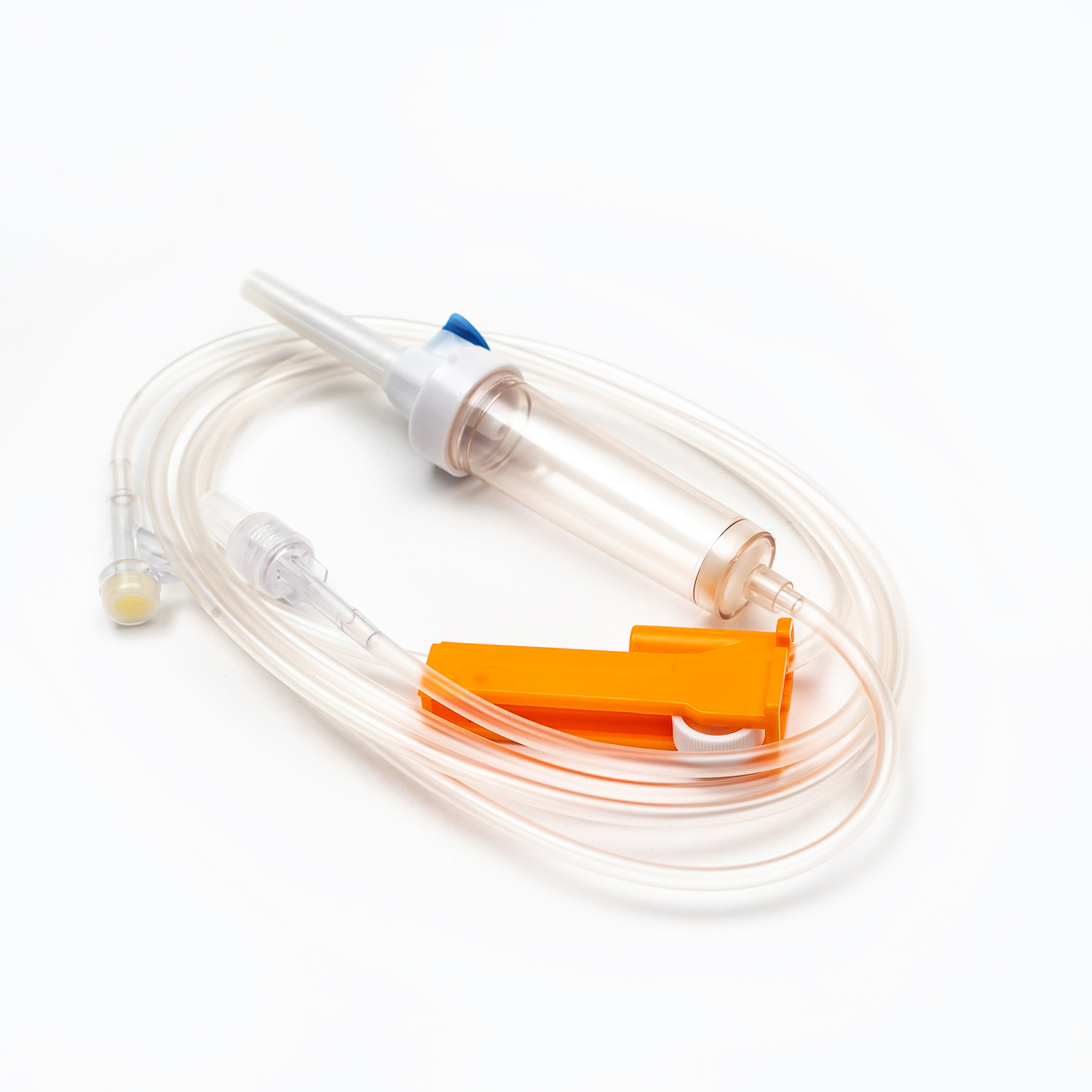
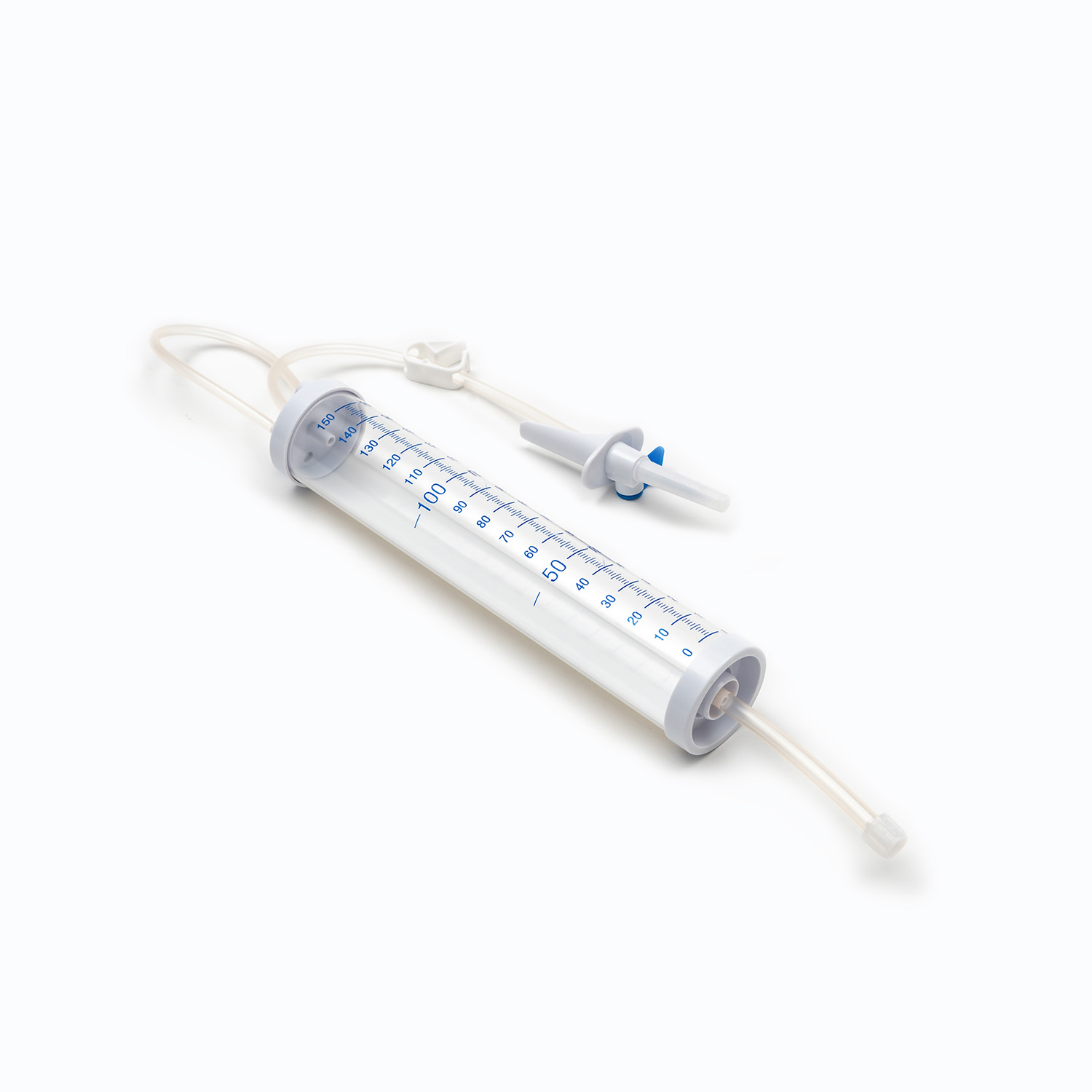
Drip Chamber
Located just below the spike, the drip chamber is a transparent, flexible bulb. This component serves several important purposes:
-
Flow Visualization: It allows healthcare professionals to visually monitor the flow of blood, observing the rate at which drops fall, which helps in calculating and maintaining the correct infusion speed.
-
Air Trap: The chamber acts as a temporary reservoir, trapping any small air bubbles that might enter from the blood bag, preventing them from reaching the patient.
-
Filter: Crucially, a filter is integrated into the bottom of the drip chamber, or just below it. This filter is typically a macropore filter with a pore size ranging from 170 to 200 microns. Its essential role is to remove any gross clots, fibrin strands, or cellular aggregates that may have formed in the stored blood. This prevents these particles from entering the patient's circulation, where they could potentially cause microemboli or pulmonary complications.
-
Macrodrip vs. Microdrip: The design of the drip chamber can also dictate the drop factor, differentiating between macrodrip and microdrip sets:
-
Macrodrip sets deliver larger drops (e.g., 10 or 15 drops/mL) and are used for most adult transfusions when a relatively rapid infusion is desired.
-
Microdrip sets produce much smaller drops (e.g., 60 drops/mL) and are typically reserved for pediatric patients, neonates, or situations where very precise and slow infusion rates are required.
-
-
Tubing
Extending from the drip chamber is a length of flexible, transparent tubing. This medical-grade tubing is designed to be kink-resistant, ensuring a continuous and unobstructed flow path for the blood. Its transparency allows for visual inspection of the blood flow and detection of any air bubbles or clots within the line.
Roller Clamp or Slide Clamp
Along the tubing, you'll find a roller clamp or a slide clamp. These mechanisms are essential for controlling the flow rate of the blood.
-
A roller clamp allows for fine-tuning of the flow rate by rolling a small wheel along the tubing to gradually compress it.
-
A slide clamp offers an on/off function or a limited number of fixed flow rates. Both types provide immediate control to start, stop, or adjust the transfusion speed as needed.
Y-Injection Site/Access Port
Many transfusion sets include a Y-injection site or access port. This port, often made of self-sealing rubber, allows for the intermittent or continuous administration of other compatible fluids, such as normal saline, or medications, without having to disconnect the main line from the patient. This is particularly useful for flushing the line before or after the transfusion.
Luer Lock Connector/Needle-Free Connector
At the distal end of the tubing is the Luer lock connector or a needle-free connector.
-
A Luer lock connector provides a secure, threaded attachment to the patient's intravenous (IV) catheter or extension set, preventing accidental disconnections.
-
Needle-free connectors are increasingly common, designed to reduce the risk of needlestick injuries for healthcare workers while maintaining a closed and sterile system for connecting to the IV access.
Air Vent (Optional but Common)
While not always present on every set, an air vent is a small, usually filtered, opening often found on sets designed for use with rigid containers (like glass bottles, though less common for blood now) or when gravity flow might create a vacuum. Its purpose is to allow air to enter the container as fluid exits, preventing a vacuum from forming and ensuring a continuous, unimpeded flow of blood. For flexible blood bags, a separate air vent isn't typically needed as the bag collapses as it empties.
Needle (if not a needle-free system)
If the set is not equipped with a needle-free connector, it will terminate in a needle (or a port for attaching one). The gauge (size) of the needle chosen for venipuncture is critical. For blood transfusions, a larger gauge needle (e.g., 18G or 20G for adults) is generally preferred to minimize shear stress on red blood cells during infusion, which can lead to hemolysis, and to facilitate a faster flow rate.
III. Types of Blood Transfusion Sets (Beyond Standard)
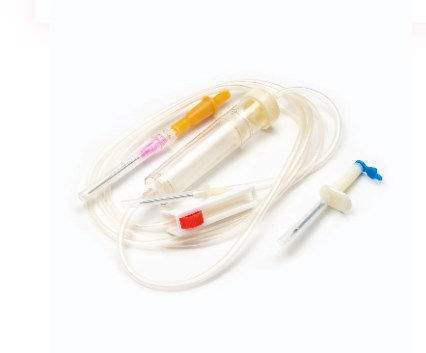
While the standard blood transfusion set serves the majority of transfusion needs, specialized clinical situations and evolving medical practices have led to the development of several advanced types of sets. These variations incorporate additional features or modifications to enhance safety, efficiency, or cater to specific patient populations and conditions.
Pediatric Blood Transfusion Sets
Pediatric blood transfusion sets are specifically designed to meet the unique requirements of infants, children, and neonates. The most significant difference lies in their microdrip chamber, which delivers a much smaller drop volume (typically 60 drops/mL) compared to the macrodrip sets used for adults. This precision is crucial for administering small, carefully titrated volumes of blood to avoid fluid overload, a significant risk in this vulnerable population. Additionally, these sets often feature smaller primary filters or are designed to be used with smaller, more controlled volumes, reflecting the lower blood volumes and slower transfusion rates typical in pediatric care.
Leukocyte Reduction Filters (Leukoreduction Filters)
Leukocyte reduction filters, often referred to as leukoreduction filters or leukoreduction sets, are a critical advancement in transfusion medicine.
-
Purpose: The primary purpose of these filters is to remove white blood cells (leukocytes) from blood products. Even though a standard filter removes larger aggregates, it does not remove the majority of leukocytes. Leukocytes in transfused blood, even in small numbers, can mediate several adverse reactions.
-
Benefits: The removal of leukocytes provides significant clinical benefits, including:
-
Preventing Febrile Non-Hemolytic Transfusion Reactions (FNHTRs): These are common transfusion reactions caused by cytokines released from donor leukocytes or by recipient antibodies reacting to donor leukocyte antigens. Leukoreduction significantly reduces their incidence.
-
Reducing the Risk of Cytomegalovirus (CMV) Transmission: CMV is a virus that can reside within leukocytes. For immunocompromised patients (e.g., transplant recipients, premature infants), CMV-safe blood (either CMV-seronegative or leukoreduced) is crucial to prevent infection.
-
Decreasing HLA Alloimmunization: Repeated exposure to donor human leukocyte antigens (HLAs) on white blood cells can lead to the formation of anti-HLA antibodies in the recipient, complicating future transfusions and organ transplantation. Leukoreduction minimizes this risk.
-
Potentially Reducing Transfusion-Related Acute Lung Injury (TRALI): While the mechanism is complex, some studies suggest leukoreduction may play a role in reducing the risk of TRALI, a severe and potentially fatal transfusion reaction.
-
-
Types: Bedside vs. Pre-storage:
-
Bedside Leukoreduction: In this method, the leukocyte reduction filter is integrated directly into the blood transfusion set and used at the patient's bedside during the transfusion. This was historically common.
-
Pre-storage Leukoreduction: This is now the more prevalent and preferred method. Blood is leukoreduced at the blood collection centerbeforestorage. This method is generally more effective at removing leukocytes, potentially reduces the accumulation of cytokines during storage, and simplifies the bedside procedure for healthcare providers. Most developed countries now have universal pre-storage leukoreduction policies.
-
Blood Warmer Sets
Blood warmer sets are used in conjunction with blood warming devices to raise the temperature of the blood product to near body temperature before infusion.
-
Integrated or Used in Conjunction: These sets are either specifically designed to fit into a blood warmer device (often featuring a coiled segment of tubing that passes through the warmer) or are used as the standard transfusion set immediately downstream from a dedicated blood warmer.
-
Purpose: Warming blood is critical in situations involving rapid, massive transfusions (e.g., trauma, major surgery) or for hypothermic patients. Infusing large volumes of cold blood rapidly can lead to profound hypothermia in the patient, which can exacerbate coagulopathy, increase the risk of cardiac arrhythmias, and impair recovery. Blood warmer sets ensure that the infused blood contributes to maintaining the patient's core body temperature.
High-Flow/Rapid Transfusion Sets
High-flow or rapid transfusion sets are specialized for situations demanding extremely quick delivery of blood products, typically in trauma situations, operating rooms, or massive transfusion protocols.
-
Design Features: These sets are characterized by larger bore tubing and sometimes larger drip chambers or simplified filter designs (though still maintaining necessary filtration) to maximize flow rates. They are often used with pressure infusion devices to mechanically push blood into the patient at speeds far greater than gravity alone could achieve.
-
Application: Their design prioritizes speed of delivery, which is paramount when a patient is hemorrhaging severely and immediate volume resuscitation and oxygen-carrying capacity restoration are life-saving.
IV. Mechanism of Action and Principles
The seemingly simple act of a blood transfusion relies on a blend of physical principles that the transfusion set expertly harnesses to ensure safe and effective delivery.
Gravity Feed
The most fundamental principle governing the flow of blood through a standard transfusion set is gravity feed. The blood bag is suspended at a height greater than the patient's intravenous access site (typically on an IV pole). This difference in height creates a hydrostatic pressure gradient. The fluid (blood) in the bag, under the influence of gravity, exerts pressure that forces it down the tubing and into the patient's vein, which has a lower pressure. The greater the height difference, the greater the hydrostatic pressure, and thus, the faster the potential flow rate. This passive flow mechanism is both simple and reliable, making it a cornerstone of intravenous fluid and blood administration.
Flow Rate Regulation
While gravity provides the motive force, precise flow rate regulation is critical to ensure that the blood is administered at the appropriate speed for the patient's condition and the blood product type. This regulation is primarily achieved through the roller clamp or slide clamp on the tubing.
-
Roller Clamp Mechanism: The roller clamp works by compressing the flexible tubing. By rolling the wheel up or down along the clamp's housing, the user can gradually increase or decrease the compression on the tubing.
-
Rolling the roller up (towards the blood bag) progressively opens the lumen of the tubing, decreasing resistance and allowing more fluid to flow, thus increasing the drip rate.
-
Rolling the roller down (away from the blood bag) gradually constricts the tubing, increasing resistance and slowing down the flow. When rolled completely down, it fully occludes the tubing, stopping the flow entirely. This precise compression allows for fine adjustments to achieve the desired drops per minute (gtt/min) or milliliters per hour (mL/hr) as prescribed.
-
Filtration Principles
Filtration is a non-negotiable safety feature of every blood transfusion set. The filters within the drip chamber, and specialized leukocyte reduction filters, operate on distinct principles:
-
Macropore (Standard) Filtration (170-200 microns): The primary filter found in the drip chamber works on a mechanical exclusion principle. Its mesh-like structure with defined pore sizes (170-200 microns) physically traps larger particles. These particles primarily include:
-
Microaggregates: Tiny clumps formed during blood storage, composed of degenerating leukocytes, platelets, and fibrin.
-
Fibrin strands: Insoluble protein fibers that can form in stored blood.
-
Clots: Small blood clots that may have formed in the bag. By removing these macroscopic contaminants, this filter prevents them from entering the patient's circulation, where they could cause pulmonary microemboli, organ damage, or activate inflammatory responses.
-
-
Leukocyte Reduction Filtration (Micropore/Submicron Filtration): Leukocyte reduction filters (typically less than 5 microns, often submicron) employ a more sophisticated combination of principles to remove white blood cells, which are much smaller than what a standard filter can trap:
-
Mechanical Sieving: The filter media contains extremely small pores that physically trap leukocytes based on their size and deformability.
-
Adhesion/Adsorption: This is a crucial mechanism. The filter material (often specially treated polymer fibers) has surface properties (e.g., charge, hydrophilicity) that cause leukocytes to adhere to the filter fibers as blood passes through. Red blood cells and plasma components, being less adherent, pass through.
-
Depth Filtration: Instead of just a flat sieve, these filters often have a tortuous, three-dimensional matrix through which the blood flows, increasing the surface area and contact time for cells to be captured.
-
These combined principles ensure that the blood delivered to the patient is not only free from large particulate matter but, in the case of leukoreduced products, also significantly depleted of white blood cells, thereby mitigating various transfusion-related complications.
V. Safety and Best Practices
The inherent risks associated with blood transfusion necessitate stringent safety protocols and adherence to best practices, many of which directly involve the proper use and handling of the blood transfusion set itself. Ensuring patient safety is paramount, and the following principles guide the safe administration of blood products.
Sterilization
Every blood transfusion set is manufactured and supplied as a sterile, single-use device. This is a non-negotiable requirement. Sterilization, typically achieved through methods like ethylene oxide gas or gamma irradiation, eliminates all microorganisms, preventing the introduction of bacteria, viruses, or fungi into the patient's bloodstream during transfusion. Healthcare providers must always verify the integrity of the sterile packaging before use and discard any set that appears to be compromised or expired.
Single-Use
The "single-use" designation is critical. Blood transfusion sets are designed for one-time use on a single patient. Reusing a set, even after attempting to clean or sterilize it, is strictly prohibited due to the impossibility of guaranteeing complete sterility and the potential for residual blood or contaminants, which could lead to severe infections, cross-contamination, or adverse reactions. After a transfusion is complete or if the set becomes compromised, it must be properly disposed of as biohazardous waste.
Filter Pore Size Importance
The presence and specific pore size of the filter within the blood transfusion set are fundamental to patient safety. As discussed, the standard 170-200 micron filter is vital for removing larger clots and aggregates that form during blood storage. Using a set without an adequate filter, or one with a damaged filter, directly exposes the patient to the risk of pulmonary microemboli and other circulatory complications. For specific clinical situations requiring leukocyte depletion, the precise sub-micron pore sizes and adsorption properties of leukocyte reduction filters are equally important to prevent immunologically mediated reactions and pathogen transmission. Always verify that the correct type of filter is present for the intended blood product and patient need.
Prevention of Air Embolism
An air embolism – the entry of air into the bloodstream – is a potentially fatal complication of intravenous infusions, including blood transfusions. The design of the transfusion set, coupled with proper technique, is crucial in its prevention.
-
Proper Priming: Before connecting the set to the patient, it must be meticulously "primed" by allowing the blood product (or saline, if starting with a flush) to completely fill the tubing, expelling all air. This involves inverting the drip chamber to fill the filter, gently squeezing the chamber to fill it appropriately, and then slowly opening the clamp to allow fluid to flow through the entire length of the tubing until all air bubbles are expelled from the distal end.
-
Secure Connections: Ensuring all connections (spike to blood bag, luer lock to IV catheter) are tight and secure prevents air from being entrained into the system.
-
Monitoring Drip Chamber: The drip chamber's function as an air trap also highlights its importance; it should be regularly monitored during transfusion.
Monitoring for Reactions
While the transfusion set itself is a delivery device, its proper function is integral to the overall safety of the transfusion process, which includes vigilant monitoring for adverse reactions. Any malfunction of the set (e.g., kinked tubing, occlusion, air in line) can impede flow and delay the observation of vital signs or the onset of a reaction. Healthcare professionals must continuously observe the patient for signs of transfusion reactions (e.g., fever, chills, rash, dyspnea, pain) during and immediately after the transfusion, ensuring the set remains patent and functional to allow for prompt intervention if a reaction occurs.
Proper Priming Techniques
As mentioned under air embolism, proper priming techniques are foundational. This involves:
-
Closing all clamps before spiking the blood bag.
-
Spiking the blood bag securely.
-
Squeezing the drip chamber to fill it to the recommended level (usually halfway).
-
Opening the roller clamp slowly to allow blood to flow through the tubing, completely removing all air.
-
Ensuring the filter in the drip chamber is fully immersed to prevent air trapping. Failure to prime correctly introduces air and can lead to serious patient harm.
Compatibility with Different Blood Components
Finally, it's essential to understand the compatibility of the transfusion set with different blood components. Standard blood transfusion sets are designed for use with all major blood components:
-
Packed Red Blood Cells (PRBCs): The most commonly transfused component, readily flows through standard sets.
-
Fresh Frozen Plasma (FFP): Once thawed, plasma transfuses well through standard sets.
-
Platelets: While platelets are delicate, standard sets with 170-200 micron filters are generally suitable. Some platelet products may benefit from specific platelet filters, but this is less common at the bedside.
-
Cryoprecipitate: This component also flows through standard sets.
However, specific components or clinical situations might warrant the specialized sets mentioned previously (e.g., leukoreduced products requiring leukoreduction filters, or rapid infusions needing high-flow sets). Critically, no medications or solutions other than normal saline (0.9% NaCl) should be infused through the same line as blood unless specifically approved and demonstrated to be compatible, as many medications and solutions (e.g., Dextrose solutions, Ringer's Lactate) can cause hemolysis or clotting of the blood product within the tubing.
VI. Complications Related to Transfusion Sets (Indirectly)
While modern blood transfusion sets are designed with safety as a priority, their improper use, manufacturing defects, or inherent limitations can indirectly lead to various complications. These issues are often preventable through meticulous attention to detail and adherence to established protocols.
Blockage/Clotting Within the Set
One of the most common complications encountered with blood transfusion sets is the blockage or clotting within the tubing or filter. This can occur due to several factors:
-
Slow Infusion Rate: If blood is infused too slowly, especially packed red blood cells which are more viscous, it can spend too much time in the tubing and begin to coagulate.
-
Inadequate Priming: Residual air bubbles or insufficient flushing can create areas where blood flow is stagnant or turbulent, promoting clot formation.
-
Improper Flushing: Failure to flush the line with normal saline before and after certain procedures (e.g., medication administration through the Y-site) can lead to residual blood clotting.
-
Mixing Incompatible Solutions: Infusing incompatible solutions or medications (e.g., Dextrose solutions, Ringer's Lactate) through the blood line can cause red blood cell agglutination or hemolysis, leading to clots that block the filter or tubing.
-
Filter Overload: In rare cases of highly clotted or aggregated blood products (which should ideally be detected and not transfused), the filter can become overwhelmed and completely blocked.
-
Kinked Tubing: Physical kinks in the tubing can impede flow, causing blood to stagnate and clot proximal to the obstruction.
A blocked set leads to cessation of blood flow, delaying critical transfusions, and requiring the discontinuation of the current set and replacement with a new one, wasting valuable blood product.
Infection Risk (if not handled aseptically)
Although the blood transfusion set is supplied sterile and designed for single-use, there is an inherent infection risk if not handled aseptically. While the set itself is sterile, contamination can occur during various stages:
-
Compromised Packaging: If the sterile packaging is torn, wet, or otherwise damaged, the sterility of the set cannot be guaranteed, and it must be discarded.
-
Improper Aseptic Technique: During setup, spiking the blood bag, priming the tubing, connecting to the IV catheter, or accessing the Y-site, breaches in aseptic technique can introduce microorganisms. Touching sterile parts of the set or allowing them to contact non-sterile surfaces directly introduces pathogens.
-
Prolonged Dwell Time: While not directly a set issue, prolonged use of a single set beyond recommended guidelines (e.g., generally no more than 4 hours for blood, or after 2-4 units of PRBCs, depending on institution policy) increases the risk of bacterial growth within the fluid path if any contamination has occurred.
-
Contaminated Y-Site Access: Improper swabbing of the Y-site injection port before accessing it can introduce skin flora or environmental bacteria into the line.
Such breaches can lead to catheter-related bloodstream infections (CRBSIs) or, more severely, bacterial contamination of the transfused blood itself, leading to septic transfusion reactions, which can be life-threatening.
Air Embolism
As highlighted in the safety section, air embolism remains a serious potential complication directly related to the proper handling of the transfusion set.
-
Incomplete Priming: The most common cause is the failure to completely purge all air from the tubing before connecting it to the patient. Small bubbles, if not removed, can coalesce into larger volumes of air.
-
Disconnected Tubing: Accidental disconnection of the transfusion set from the IV catheter while the IV line is patent and the patient's arm is elevated can allow air to be entrained into the vein, especially if there's negative intrathoracic pressure (e.g., during inspiration).
-
Empty Blood Bag: Running the blood bag completely dry without clamping the line can allow air from the empty bag to enter the tubing.
-
Faulty Connections: Loose Luer lock connections or cracks in the tubing, though rare, could potentially allow air ingress.
The severity of an air embolism depends on the volume of air and the speed of entry. Small amounts may be asymptomatic, but larger volumes can lead to sudden shortness of breath, chest pain, cyanosis, hypotension, and even cardiac arrest by forming an air lock in the right ventricle, obstructing pulmonary blood flow. Vigilant observation, secure connections, and meticulous priming are essential to prevent this.
VII. Innovations and Future Trends
The field of transfusion medicine is continuously evolving, driven by the desire to enhance patient safety, optimize blood product utilization, and improve efficiency. While the core design of blood transfusion sets has remained largely consistent for decades, significant innovations are emerging, and future trends point towards increasingly smart, integrated, and patient-centric systems.
Integrated Smart Systems
The most significant trend is the development and integration of smart systems that bring automation, data capture, and real-time monitoring directly to the point of care. These systems often involve:
-
Barcode Technology and RFID: Integration of 2D barcodes on patient wristbands, blood bags, and transfusion sets, coupled with Radio-Frequency Identification (RFID) tags, allows for automated verification at multiple checkpoints. Handheld scanners or RFID readers can confirm "right patient, right blood product" at the bedside, significantly reducing human error in patient identification and cross-matching. This also enables seamless tracking of the blood product from donation to transfusion, enhancing traceability for hemovigilance.
-
Automated Verification and Documentation: Smart systems can electronically guide clinicians through transfusion protocols, prompt for vital sign checks, and automatically document every step of the transfusion process. This reduces manual charting errors, ensures compliance with hospital policies, and provides a comprehensive electronic record for audit and quality improvement.
-
Real-time Monitoring: Future sets might incorporate micro-sensors that continuously monitor blood flow rate, detect air bubbles, or even subtle changes in blood characteristics (e.g., temperature changes indicating a reaction). This data could be wirelessly transmitted to electronic health records (EHRs), alerting healthcare providers to potential issues immediately.
Improved Filtration Technology
While leukocyte reduction filters have been a major advancement, research continues to refine filtration technologies:
-
Enhanced Leukocyte Reduction: Further improvements in filter materials and pore design aim for even more efficient and consistent leukocyte removal, potentially reducing residual inflammatory mediators and further minimizing risks of FNHTRs and other immune-mediated complications.
-
Pathogen Reduction Filters: Beyond leukocytes, ongoing research explores filters capable of removing or inactivating a broader spectrum of pathogens (bacteria, viruses, parasites) directly at the bedside. While pathogen inactivation technologies for blood products are typically donebeforestorage at blood centers, a robust bedside filtration solution could offer an additional layer of safety, especially for emerging infectious threats.
-
Universal Filters: The development of more versatile filters that can effectively remove a wider range of unwanted components (e.g., microaggregates, activated platelets, certain inflammatory cytokines) while preserving the integrity and function of the transfused cells is an area of active investigation.
Enhanced Safety Features
Beyond smart system integration, physical enhancements to the sets themselves are continually being developed:
-
Anti-Kink and Anti-Occlusion Designs: New tubing materials and designs that are even more resistant to kinking or collapse, ensuring uninterrupted flow, are being explored. Some concepts include integrated flow sensors that alarm if an occlusion is detected.
-
Advanced Needle-Free Connectors: Continued innovation in needle-free access ports focuses on minimizing dead space, preventing bacterial ingress, and improving ease of use for healthcare professionals.
-
Closed-System Blood Sampling: Systems that allow for blood sampling directly from the transfusion line without exposing the system to the external environment further reduce contamination risk and blood wastage.
-
Integrated Warming Elements: While separate blood warmers are common, future designs might see more compact, disposable, or even integrated warming elements within the transfusion set itself, particularly beneficial for rapid transfusions in emergency settings.
More Compact and User-Friendly Designs
The push for greater efficiency and ease of use in busy clinical environments is leading to:
-
Miniaturization: Efforts to make transfusion sets more compact, lighter, and easier to handle, especially for field use (e.g., military medicine, emergency medical services).
-
Simplified Priming Mechanisms: Designs that simplify or automate the priming process, further reducing the chance of air embolism and improving workflow.
-
Ergonomic Enhancements: Improvements in the design of clamps, spikes, and connectors to make them more intuitive and less prone to user error, thereby enhancing the overall user experience for healthcare providers.
These innovations highlight a future where blood transfusion sets are not just conduits but active participants in ensuring the highest levels of safety, precision, and efficiency in patient care.
VIII. Conclusion
The blood transfusion set, often perceived as a simple piece of plastic tubing, is in reality a sophisticated and indispensable medical device. From its rudimentary beginnings centuries ago to the highly engineered, sterile, and often "smart" systems of today, its evolution mirrors the advancements in transfusion medicine itself.
This device serves a singular, life-sustaining purpose: to safely and efficiently transfer blood and blood components from donor to recipient. Every component, from the spike and drip chamber with its vital filter, to the tubing and clamps, is meticulously designed to ensure controlled flow and prevent the passage of harmful particulate matter and air. Specialized sets, such as those for pediatric patients, leukocyte reduction, blood warming, and rapid transfusions, highlight the adaptability of this core technology to diverse clinical needs and stringent safety requirements.
The continuous innovation in blood transfusion sets, with trends leaning towards integrated smart systems, improved filtration, and enhanced safety features, underscores the ongoing commitment to patient well-being. These future advancements promise even greater precision, reduced human error, and a proactive approach to potential complications.
Ultimately, the blood transfusion set is more than just a conduit; it's a critical link in the chain of care that saves countless lives daily, supporting patients through trauma, chronic illness, and complex medical procedures. Its role, though often behind the scenes, remains absolutely fundamental to modern healthcare, bridging the gap between a life-giving donation and a patient in need.
-



 English
English Français
Français русский
русский Español
Español