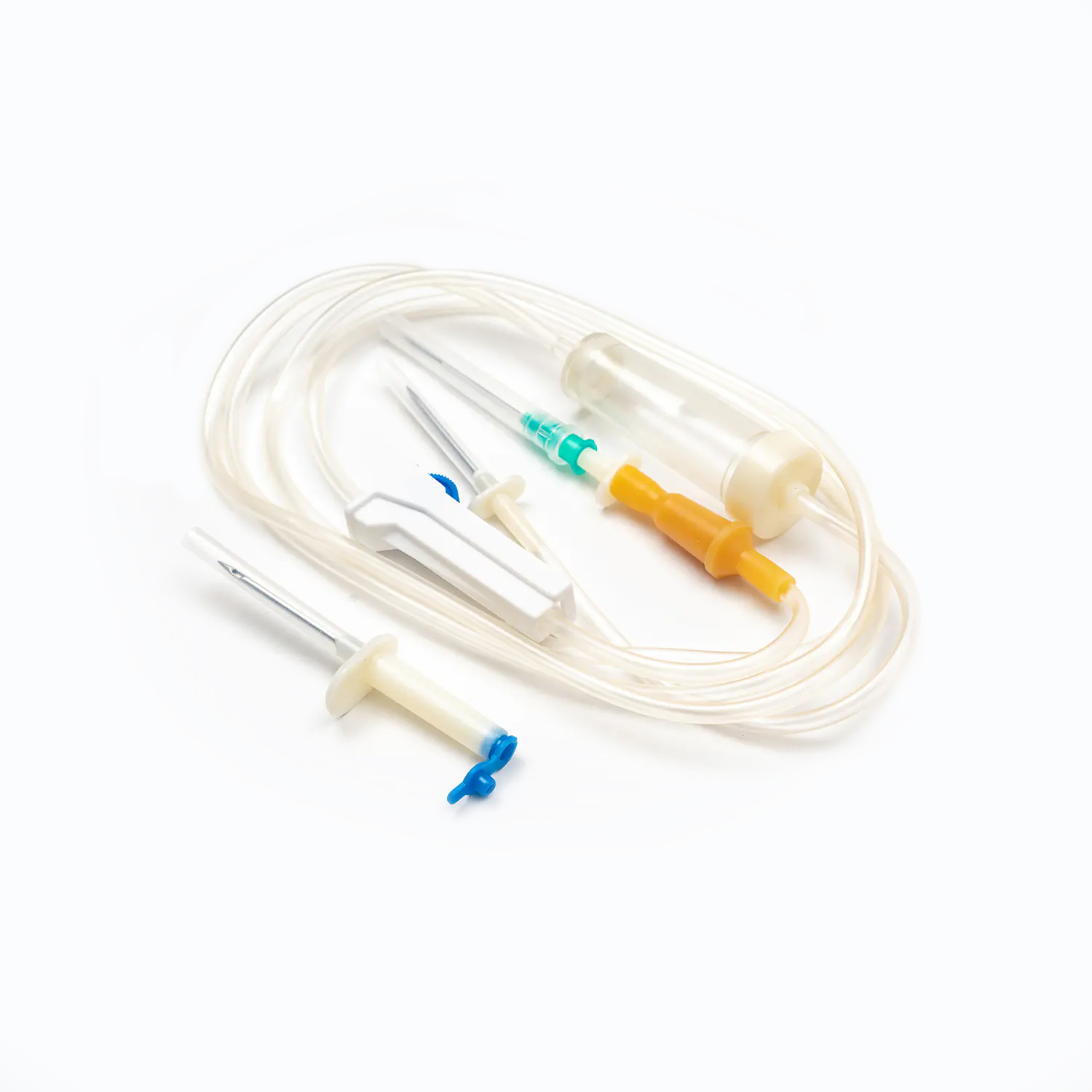
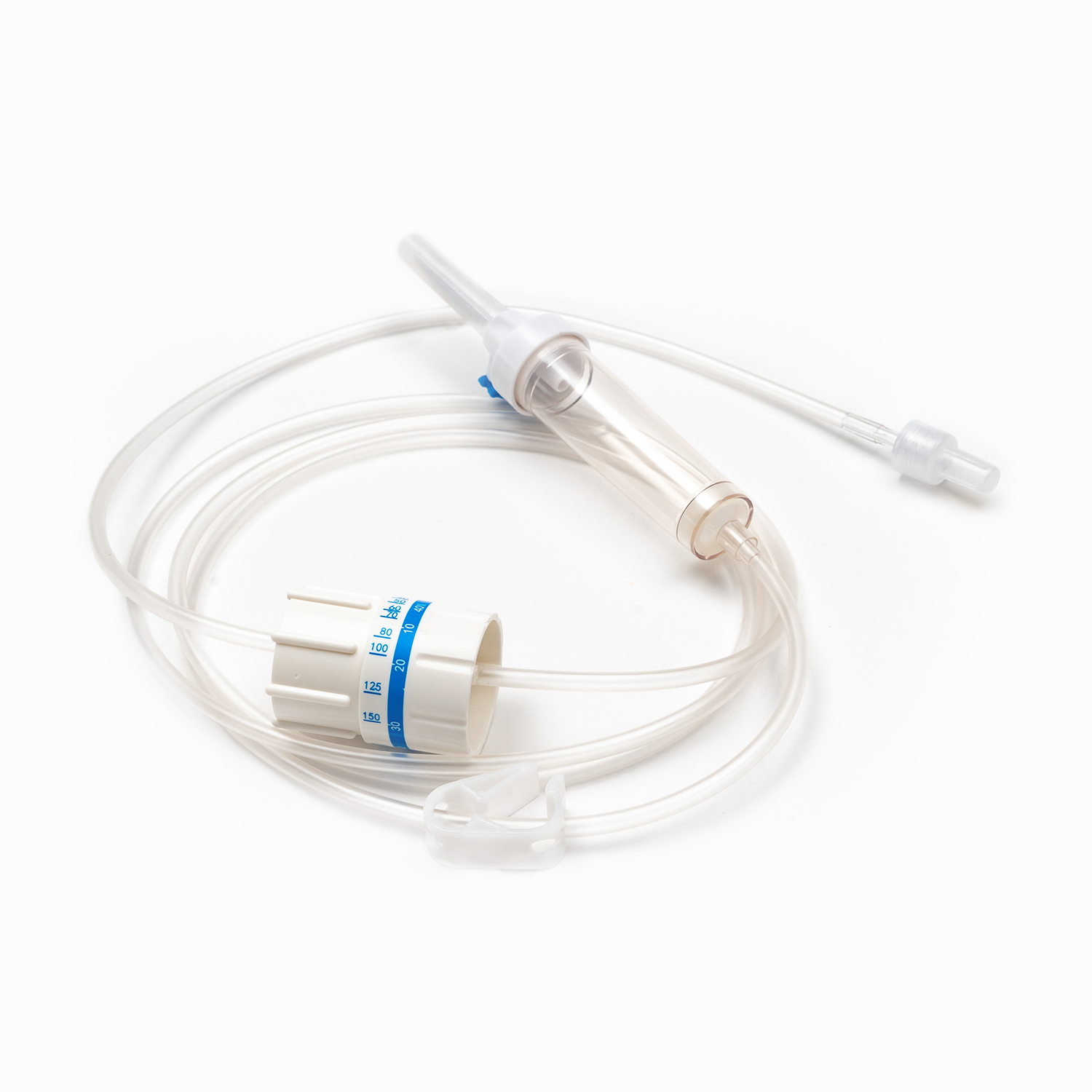
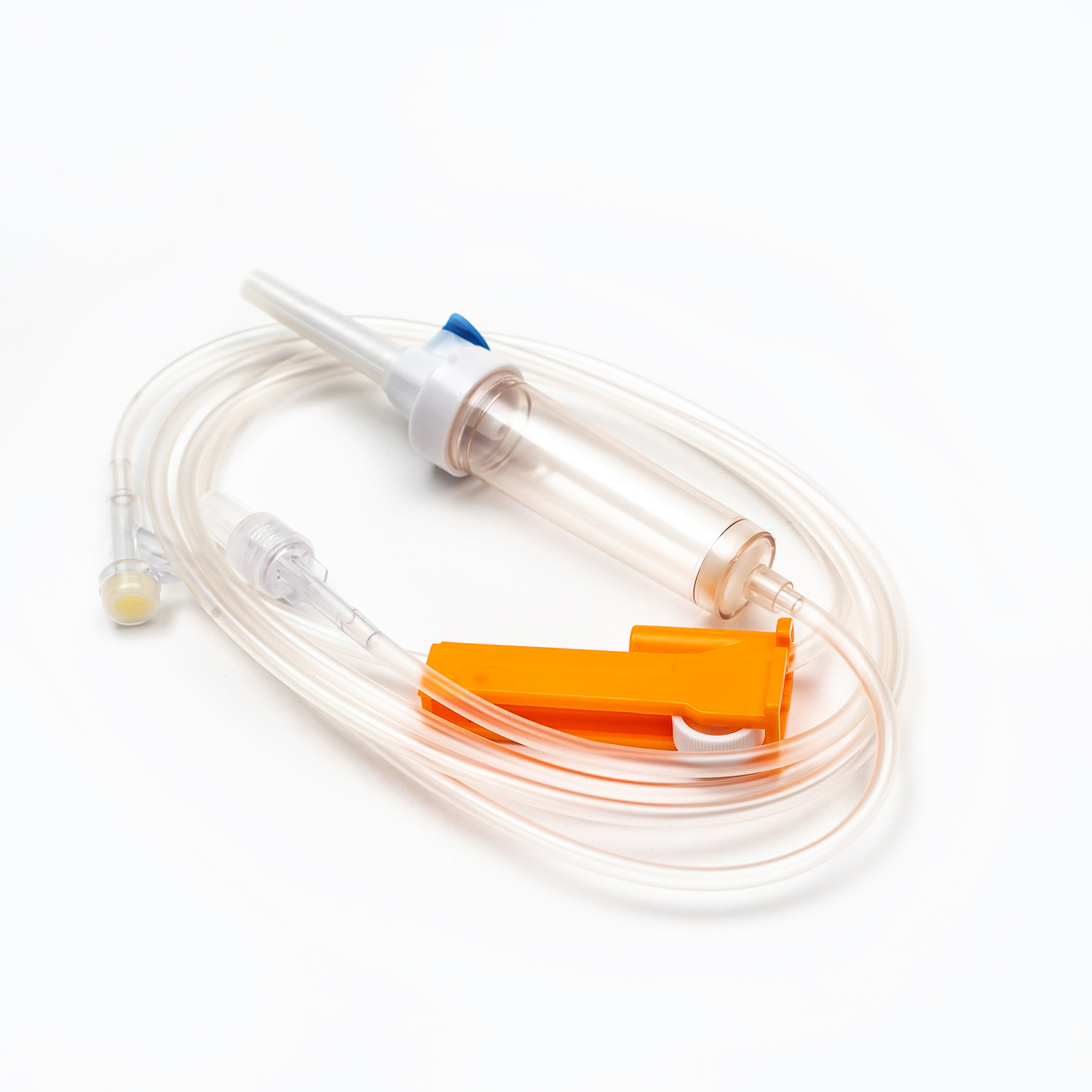
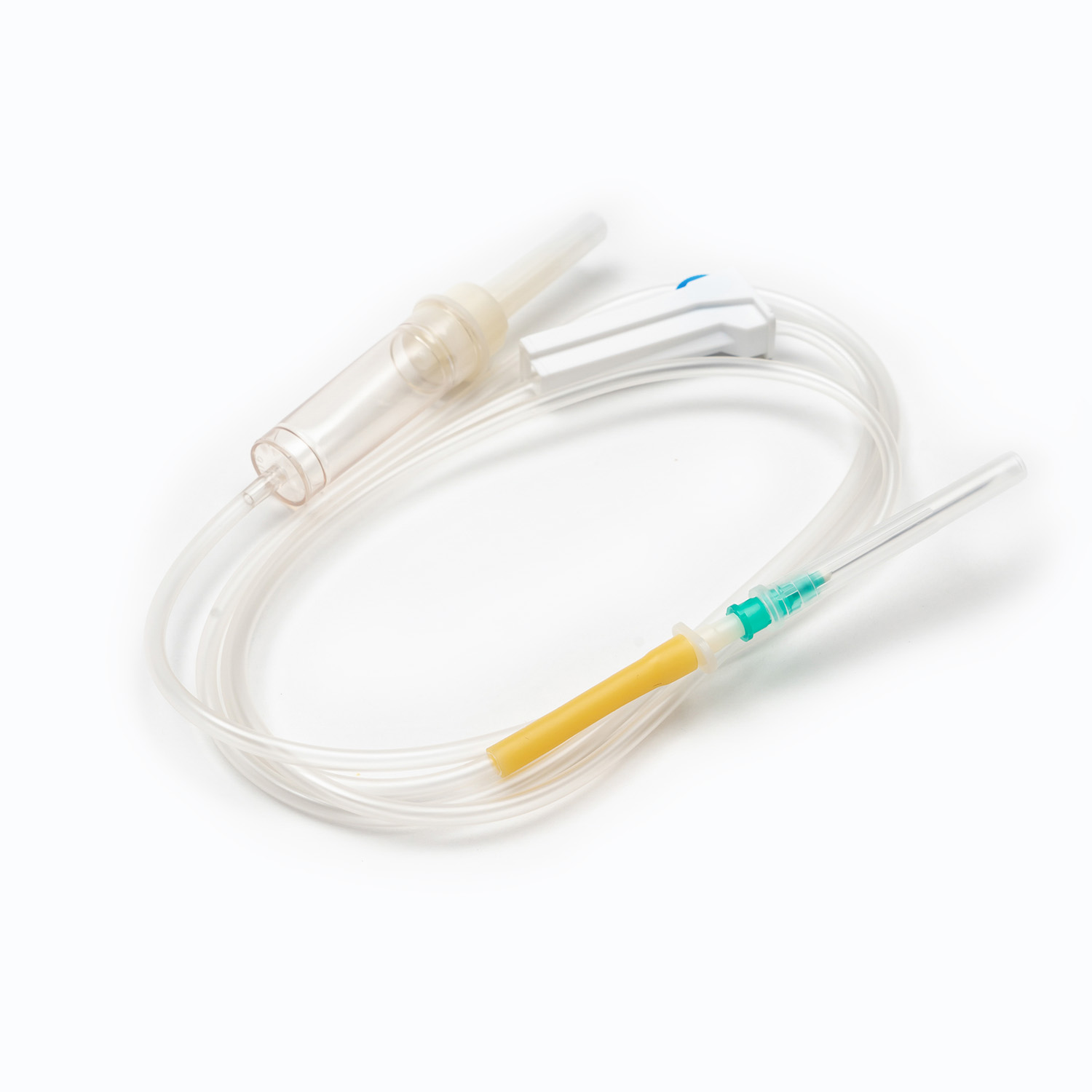
Blood Transfusion Set vs. IV Infusion Set: What Are the Main Differences?
Feb 01,2026
In clinical practice, both Blood Transfusion Set and IV infusion set are essential medical devices used to deliver fluids into a patient’s bloodstream. However, they are designed for different medical purposes, safety requirements, and regulatory standards.
Understanding Blood Transfusion Set vs. IV Infusion Set: What Are the Main Differences? is critical for healthcare providers and medical buyers who need to ensure patient safety and regulatory compliance.
Purpose and Clinical Application
Different Intended Uses
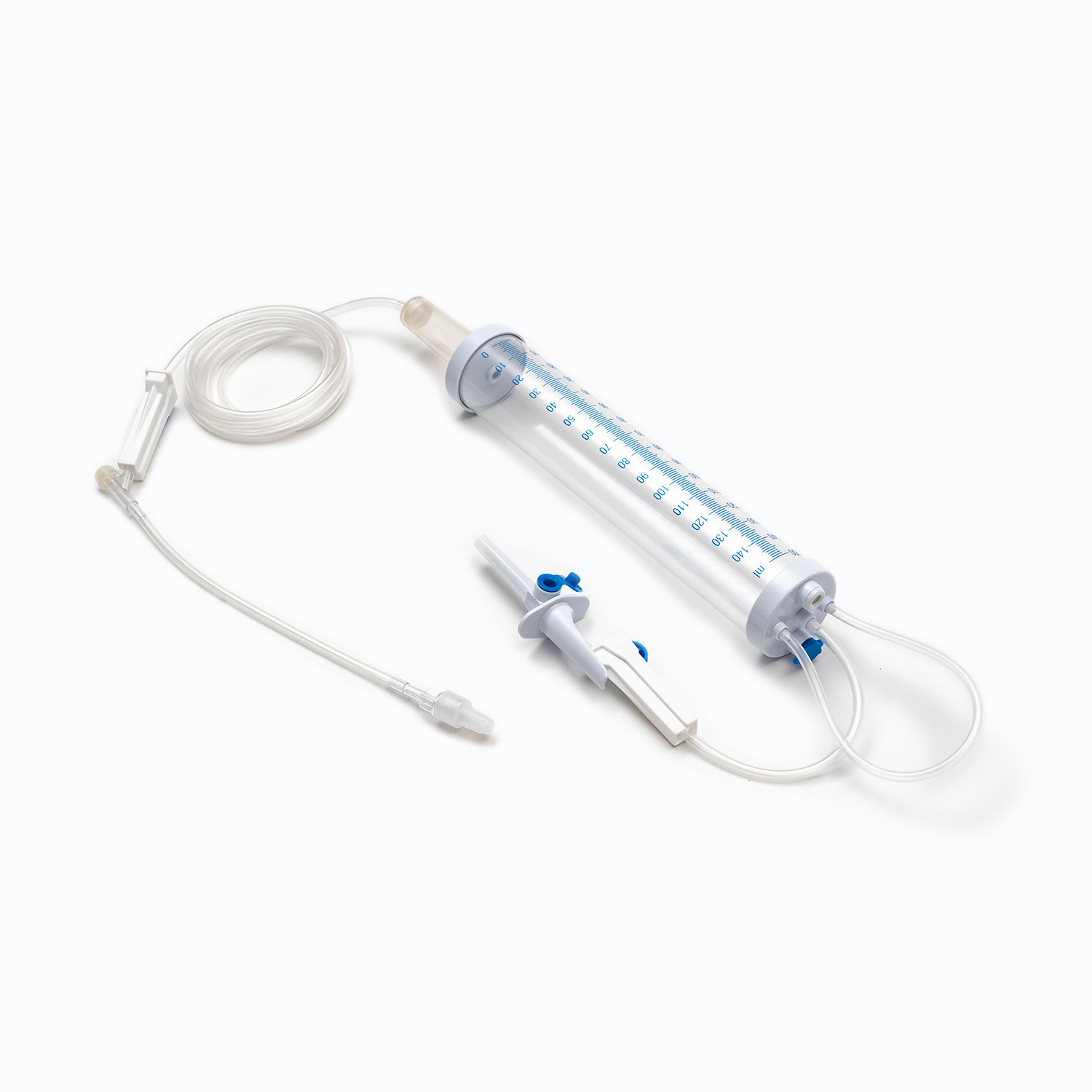
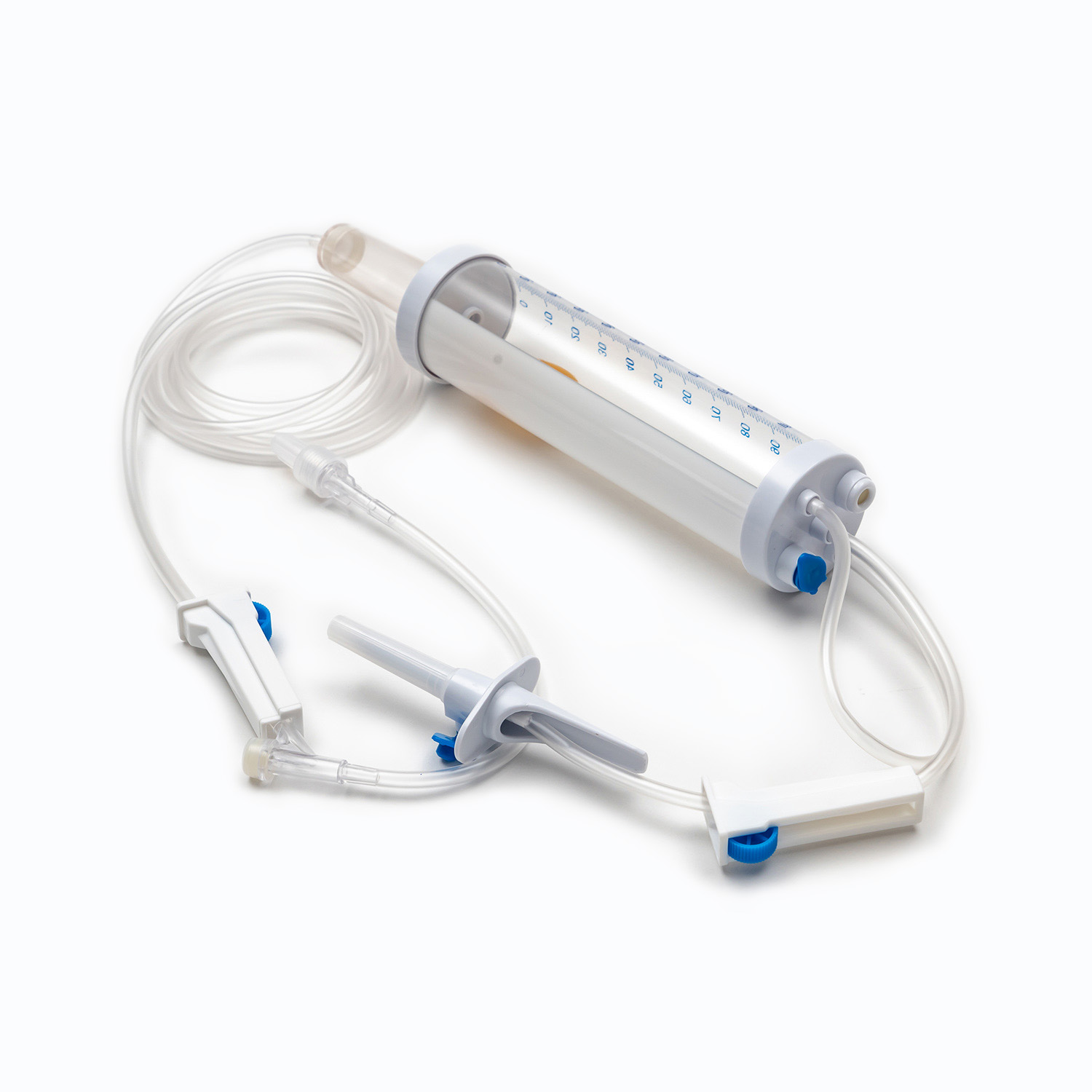
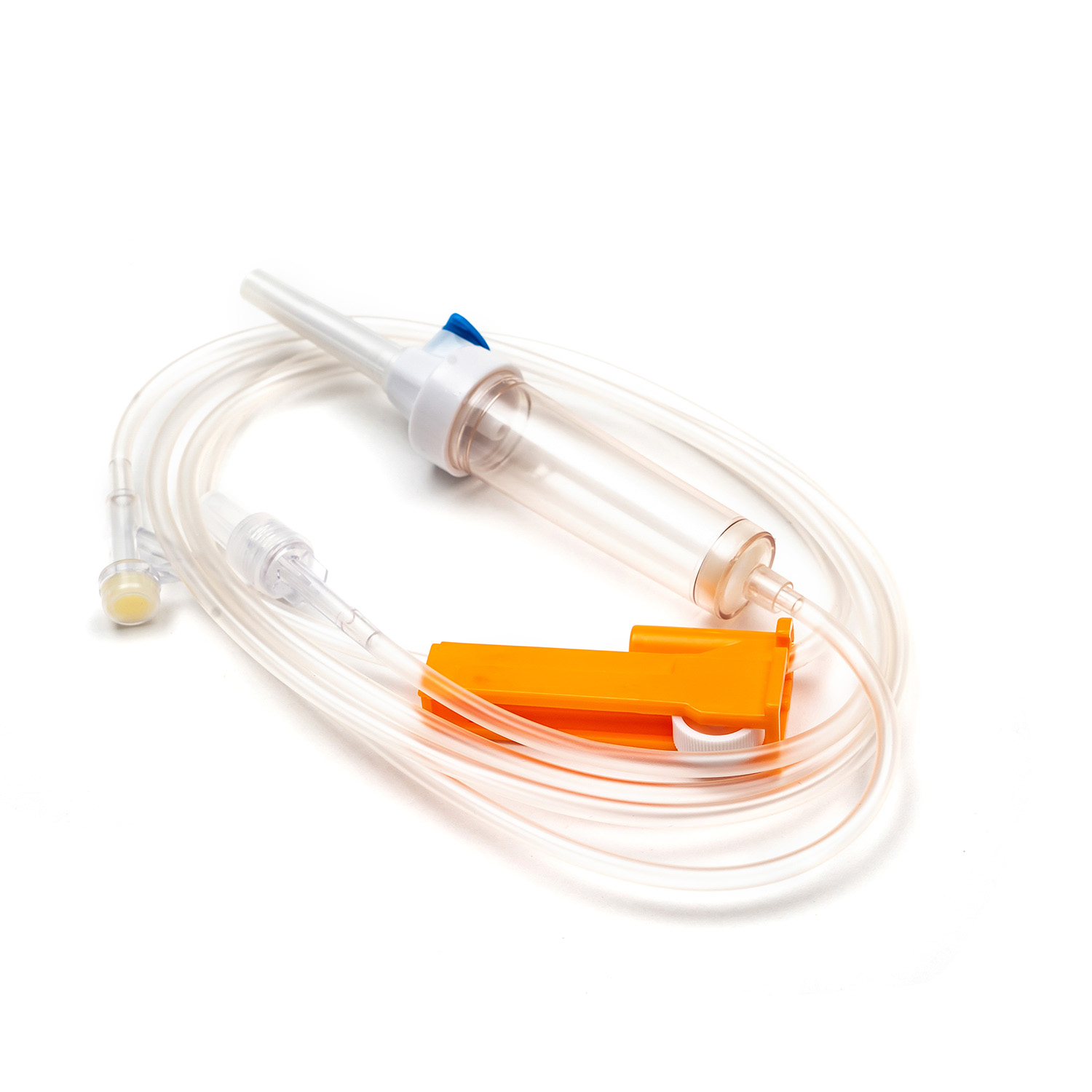
A Blood Transfusion Set is specifically designed for the transfusion of whole blood and blood components, while an IV infusion set is intended for delivering medications, saline, or nutritional fluids. This fundamental difference determines where and how each device should be used in clinical settings.
- Blood Transfusion Set is used in emergency rooms, operating theaters, ICUs, and hematology departments.
- IV infusion set is commonly used in general wards and outpatient treatments.
| Aspect | Blood Transfusion Set | IV Infusion Set |
| Primary Use | Blood and blood components | Medications and fluids |
| Clinical Application | Transfusion-specific scenarios | General infusion therapy |
Filter Design and Safety Features
Built-in Blood Filtration
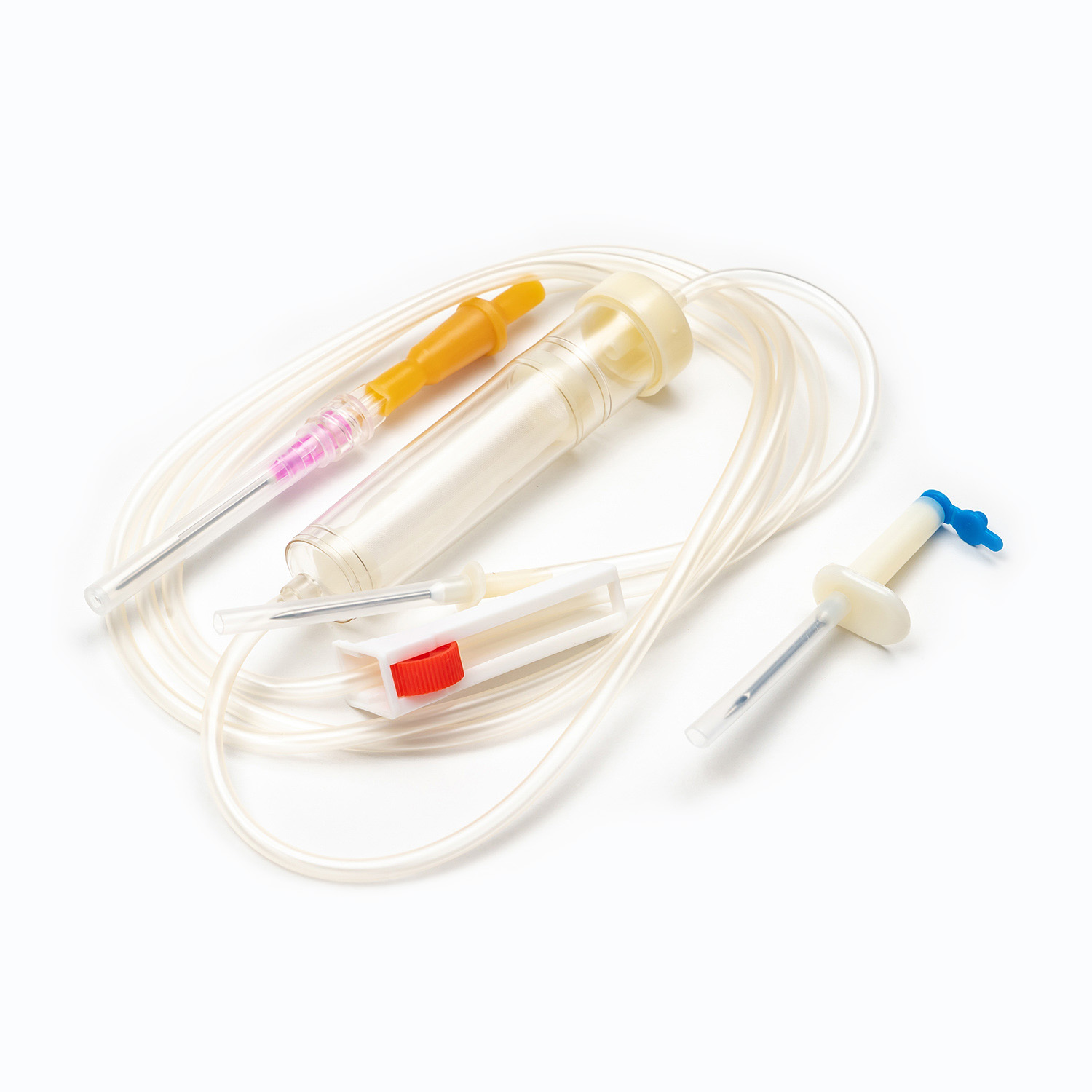
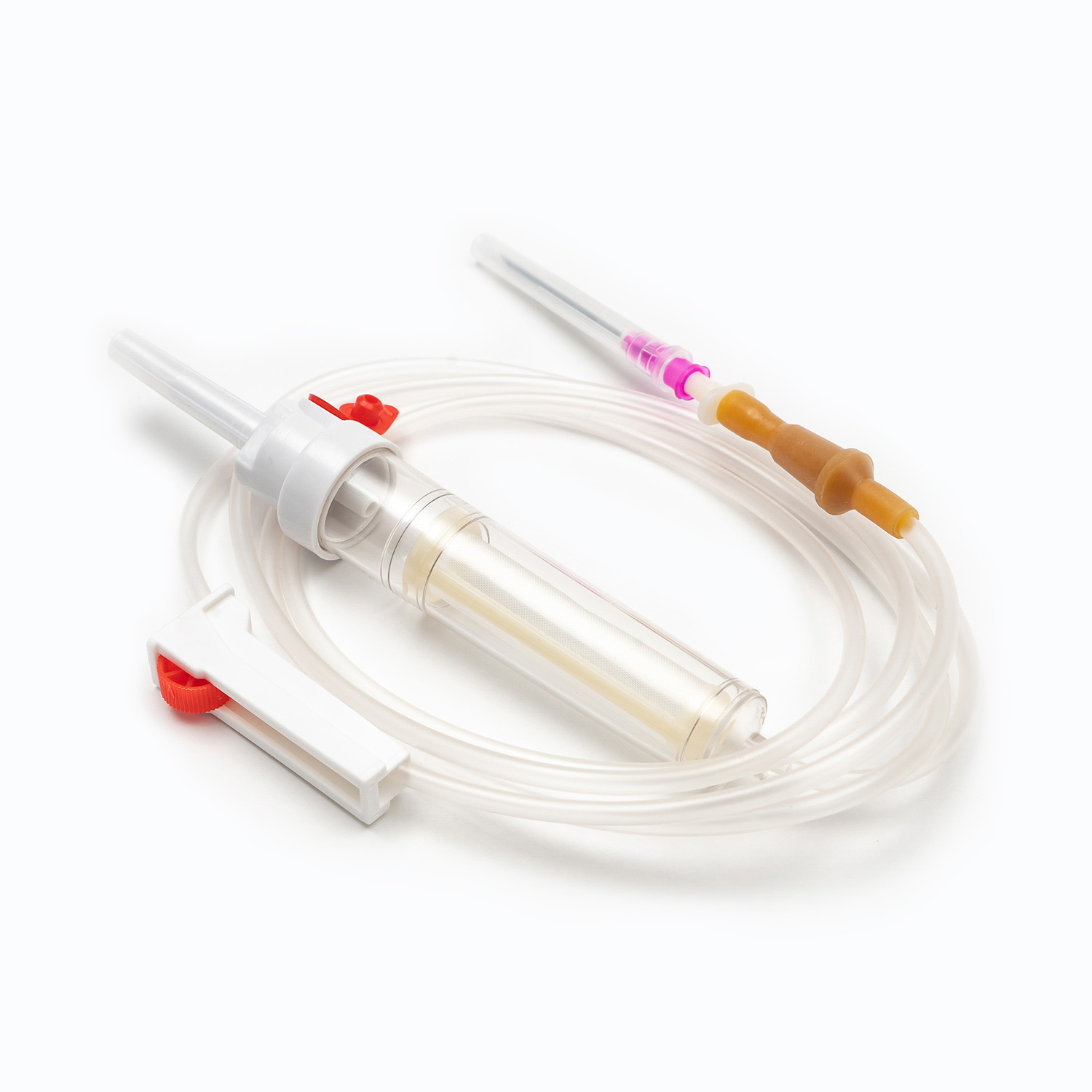
One key factor in understanding what is a Blood Transfusion Set and how does it work in clinical use is its integrated blood filter. This filter removes clots and aggregates during transfusion, which an IV infusion set cannot reliably do.
- Blood Transfusion Set includes a standard blood filter for patient safety.
- IV infusion set typically lacks blood-specific filtration.
| Aspect | Blood Transfusion Set | IV Infusion Set |
| Filter Type | 170–260 micron blood filter | No blood filter |
| Safety Level | High for transfusion use | Standard infusion safety |
Tubing Material and Flow Characteristics
Blood Compatibility
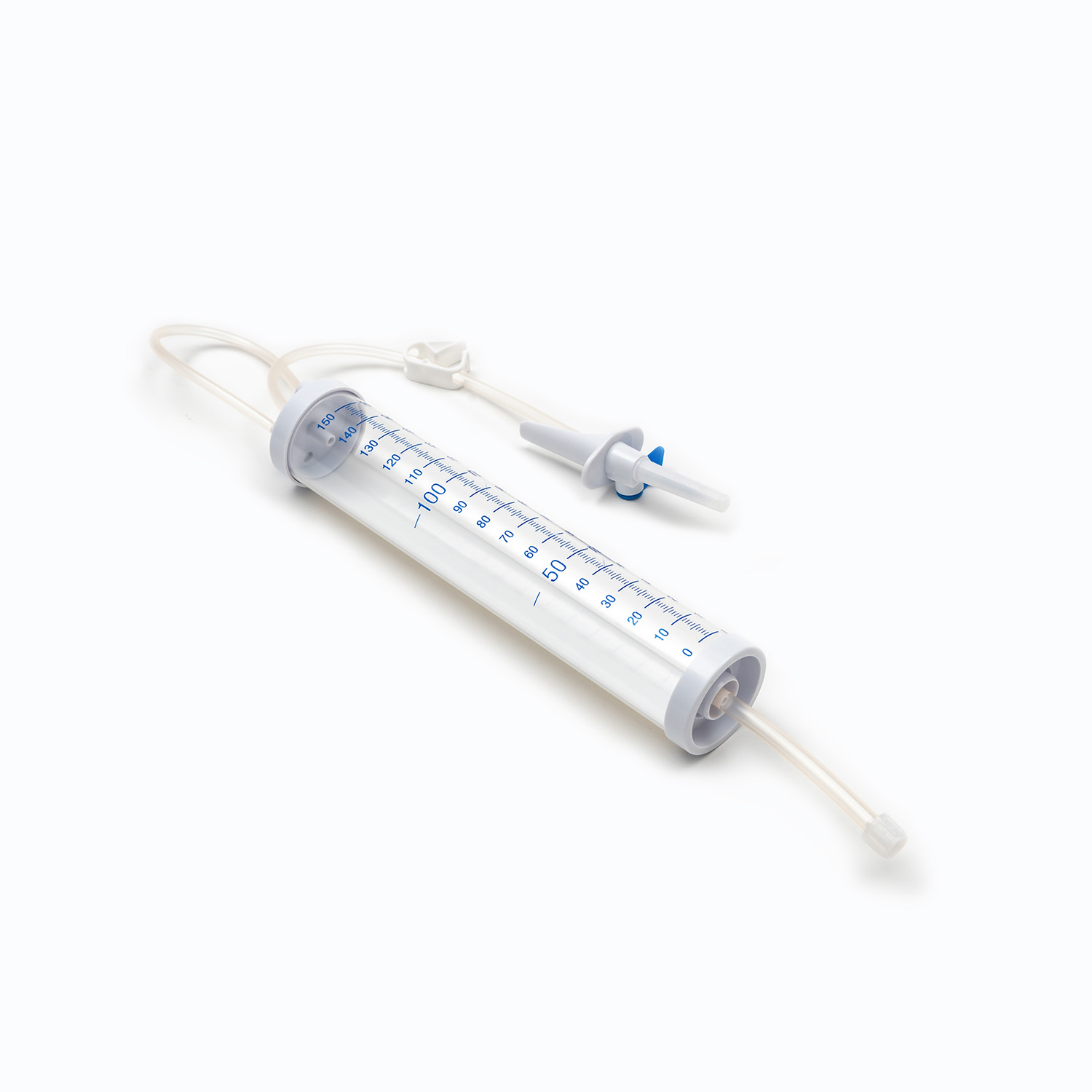
The tubing of a Blood Transfusion Set is designed to maintain blood integrity and reduce the risk of hemolysis. IV infusion sets, while suitable for fluids, are not optimized for blood viscosity and cellular protection.
- Blood Transfusion Set supports controlled blood flow.
- IV infusion set supports rapid or standard fluid infusion.
| Aspect | Blood Transfusion Set | IV Infusion Set |
| Tubing Design | Optimized for blood | Optimized for fluids |
| Risk of Hemolysis | Low | Higher if used for blood |
Standards, Certifications, and Compliance
Regulatory Requirements
When considering how to choose the right Blood Transfusion Set for hospital or clinical applications, regulatory compliance is a critical factor. Blood transfusion devices are subject to stricter standards than IV infusion sets.
- Blood Transfusion Set must comply with transfusion-related safety standards.
- IV infusion set follows general infusion device regulations.
| Aspect | Blood Transfusion Set | IV Infusion Set |
| Common Standards | ISO 1135, ISO 13485 | ISO 8536 |
| Regulatory Focus | Blood safety | Infusion safety |
Cost and Buyer Considerations
Procurement Perspective
Understanding what should buyers consider when sourcing a Blood Transfusion Set from manufacturers requires balancing cost, safety, and compliance. While blood transfusion sets are more expensive, their specialized design is essential for transfusion procedures.
- Blood Transfusion Set prioritizes safety and certification.
- IV infusion set prioritizes cost efficiency and volume usage.
| Aspect | Blood Transfusion Set | IV Infusion Set |
| Unit Cost | Higher | Lower |
| Buyer Focus | Quality and compliance | Cost and availability |
Conclusion
The comparison of Blood Transfusion Set vs. IV Infusion Set clearly shows that these devices serve different clinical purposes and are not interchangeable. Understanding the design, safety features, and regulatory requirements helps healthcare providers make informed decisions and ensures patient safety.
Frequently Asked Questions
What is a Blood Transfusion Set and how does it work in clinical use?
A Blood Transfusion Set delivers blood or blood components through a filtered system that removes clots and ensures safe transfusion into the patient’s bloodstream.
Can an IV infusion set be used for blood transfusion?
No, IV infusion sets lack blood-specific filters and are not designed to safely handle blood products.
How to choose the right Blood Transfusion Set for hospital or clinical applications?
Hospitals should consider filtration quality, tubing design, compatibility with blood bags, and compliance with international standards.
What are the key safety standards and certifications for a Blood Transfusion Set?
Key standards include ISO 1135 and ISO 13485, along with region-specific regulatory approvals.
What should buyers consider when sourcing a Blood Transfusion Set from manufacturers?
Buyers should focus on product quality, manufacturing consistency, regulatory compliance, and supplier reliability.



 English
English Français
Français русский
русский Español
Español