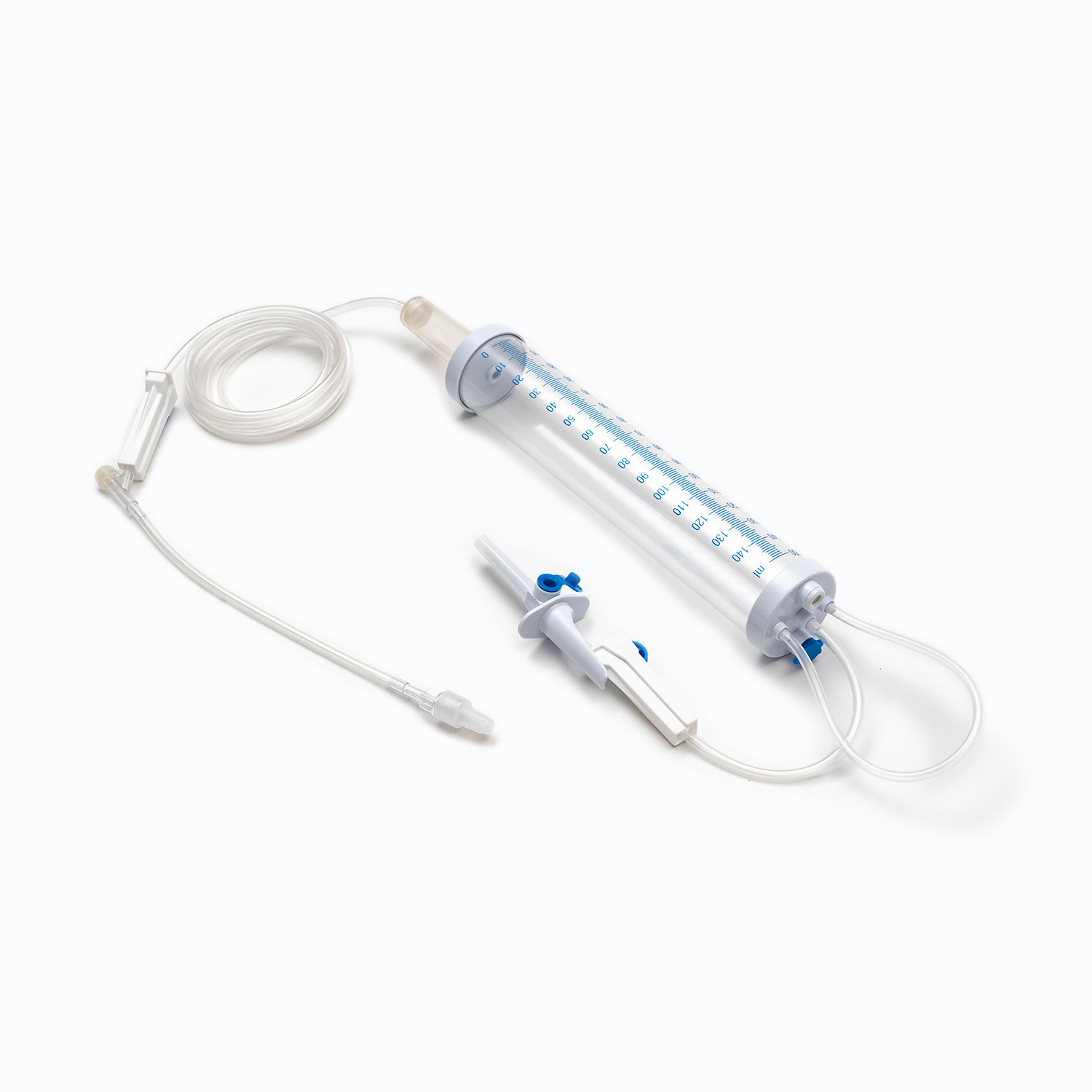
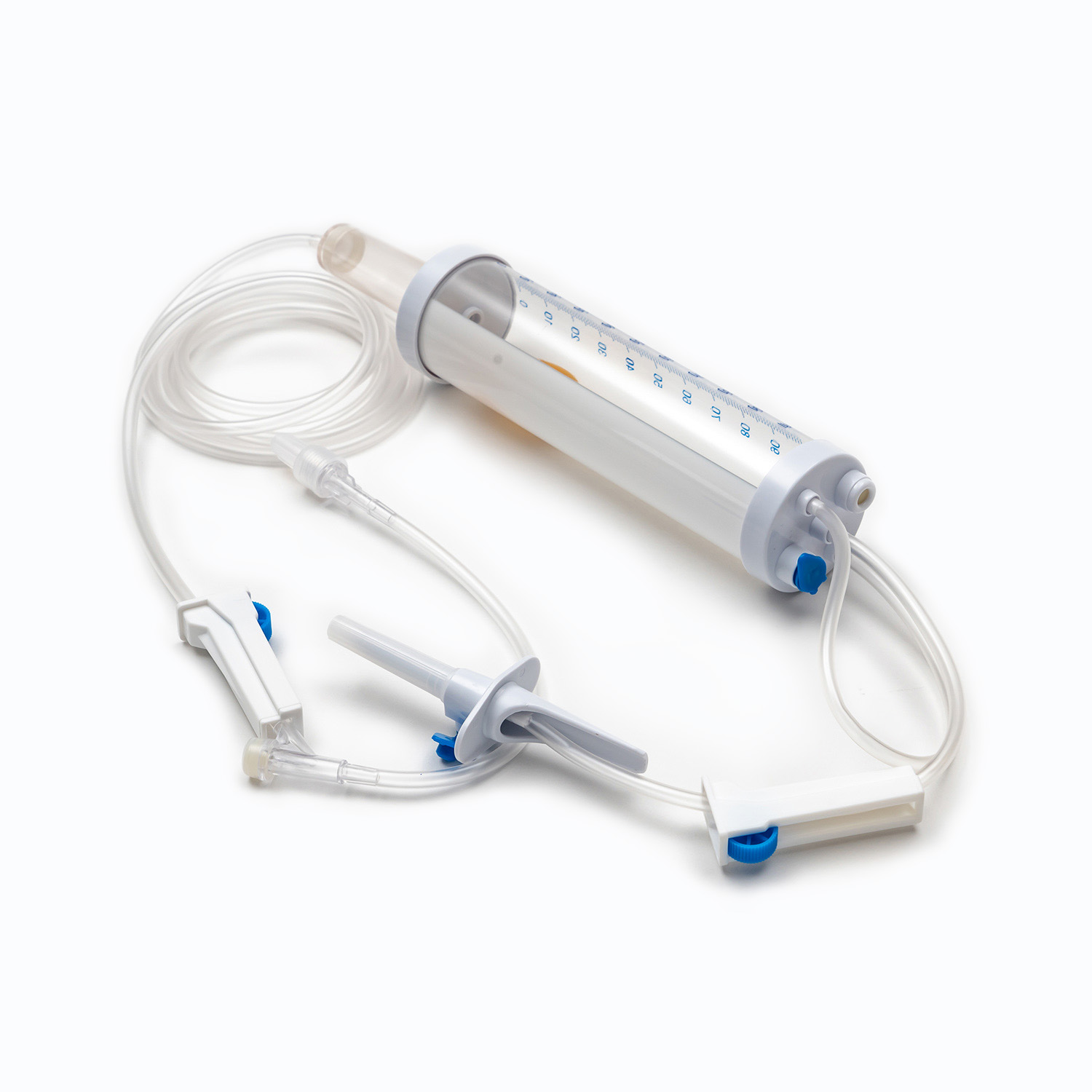
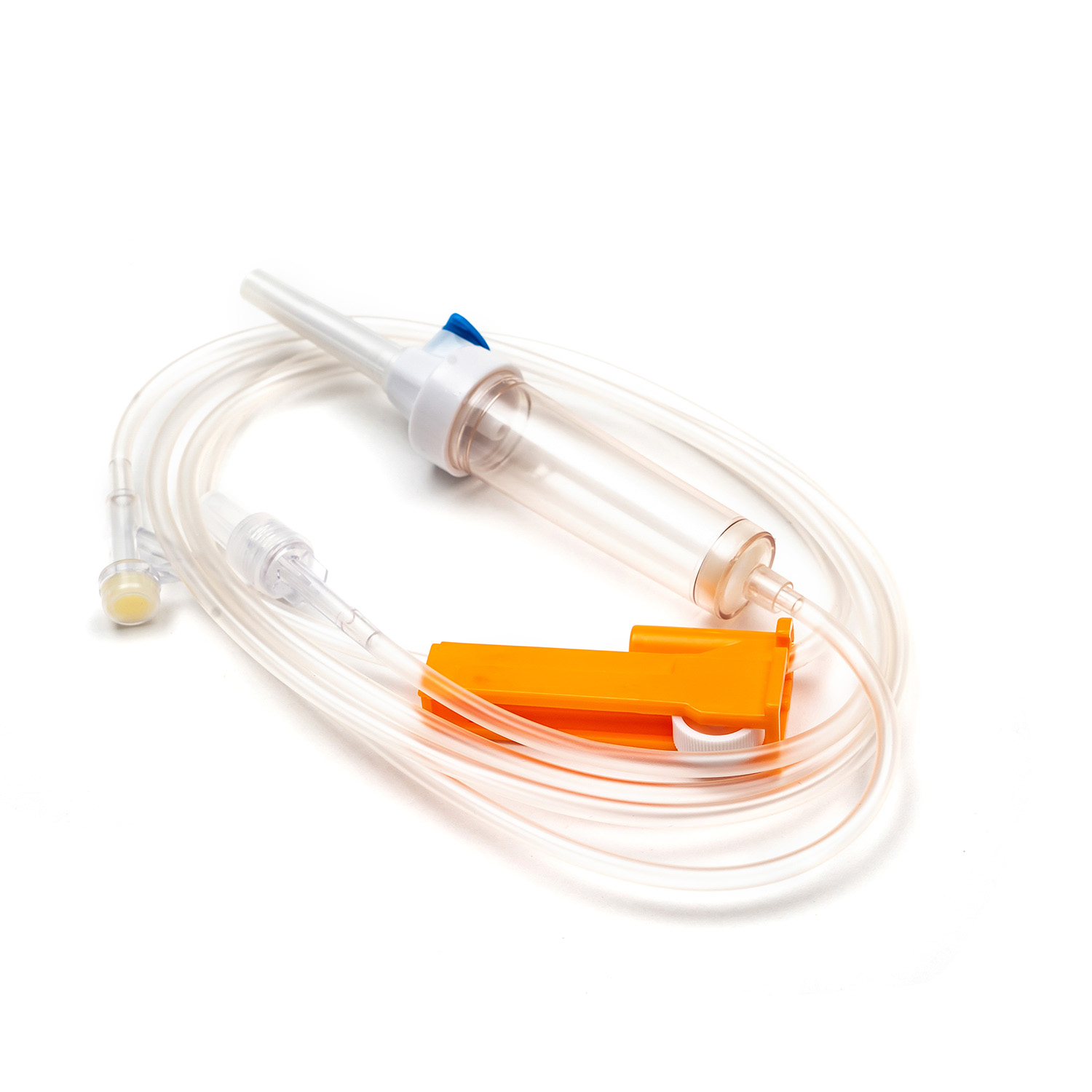
Disposable Infusion Sets: Key Equipment for Medical Infusion Safety and Efficiency
Oct 08,2025
In modern healthcare, disposable infusion sets are one of the most widely used basic medical devices in clinical treatment. With the continuous improvement of medical and health standards and the growing awareness of patient safety, the design, manufacturing, and usage standards of these products have undergone continuous innovation and improvement. They not only affect the accuracy and stability of drug delivery, but also directly impact the safety of the infusion process and the patient experience.
Definition and Structural Components of Disposable Infusion Sets
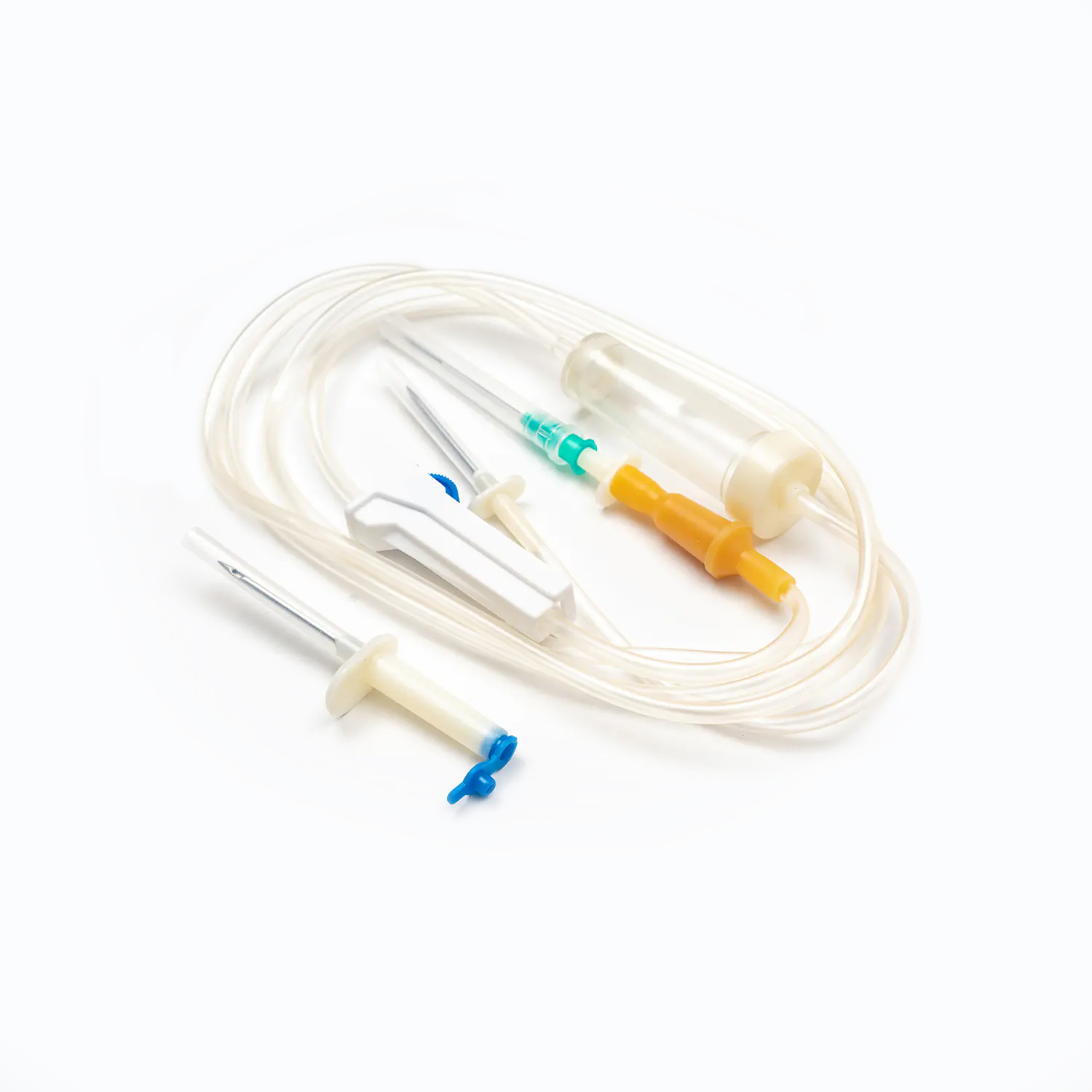
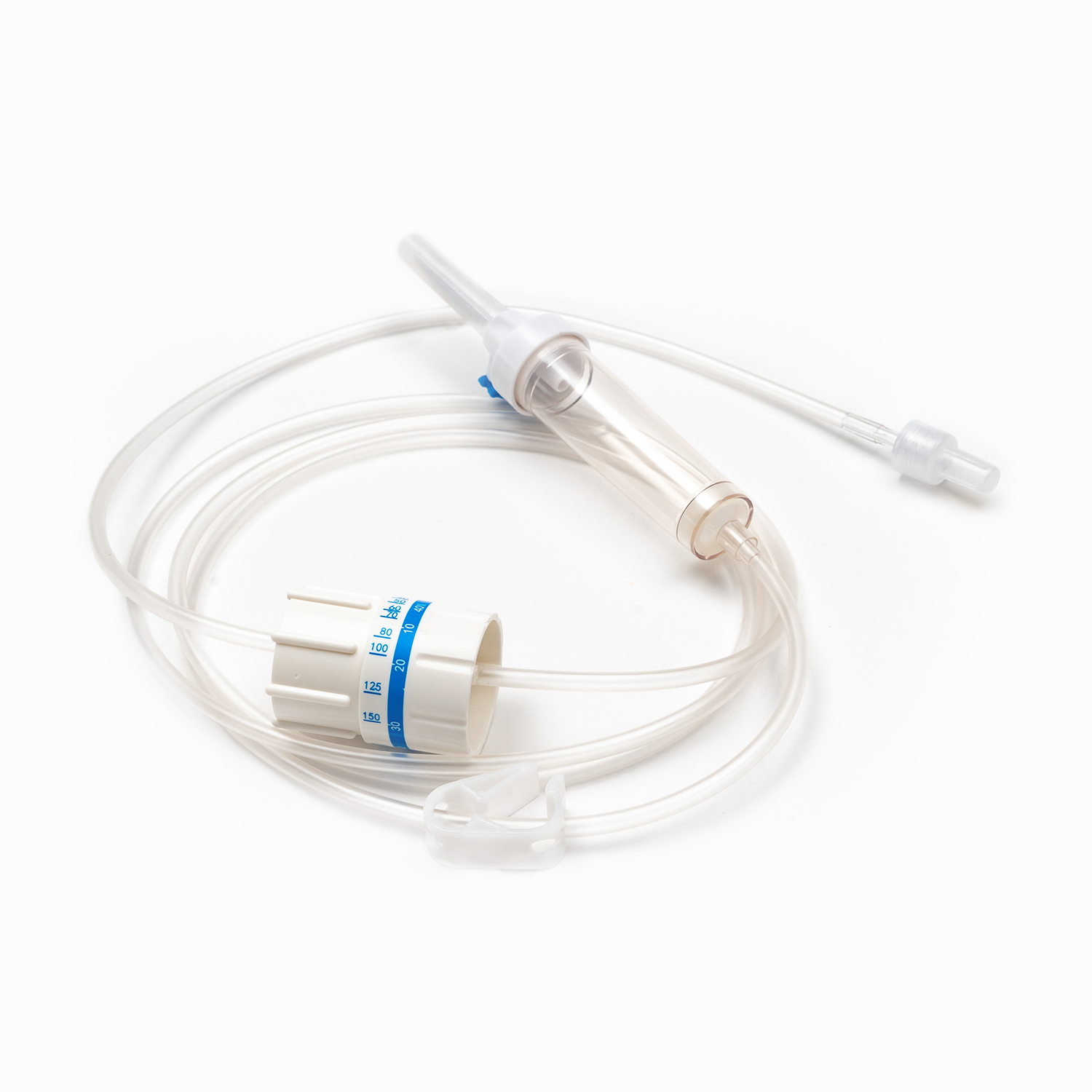
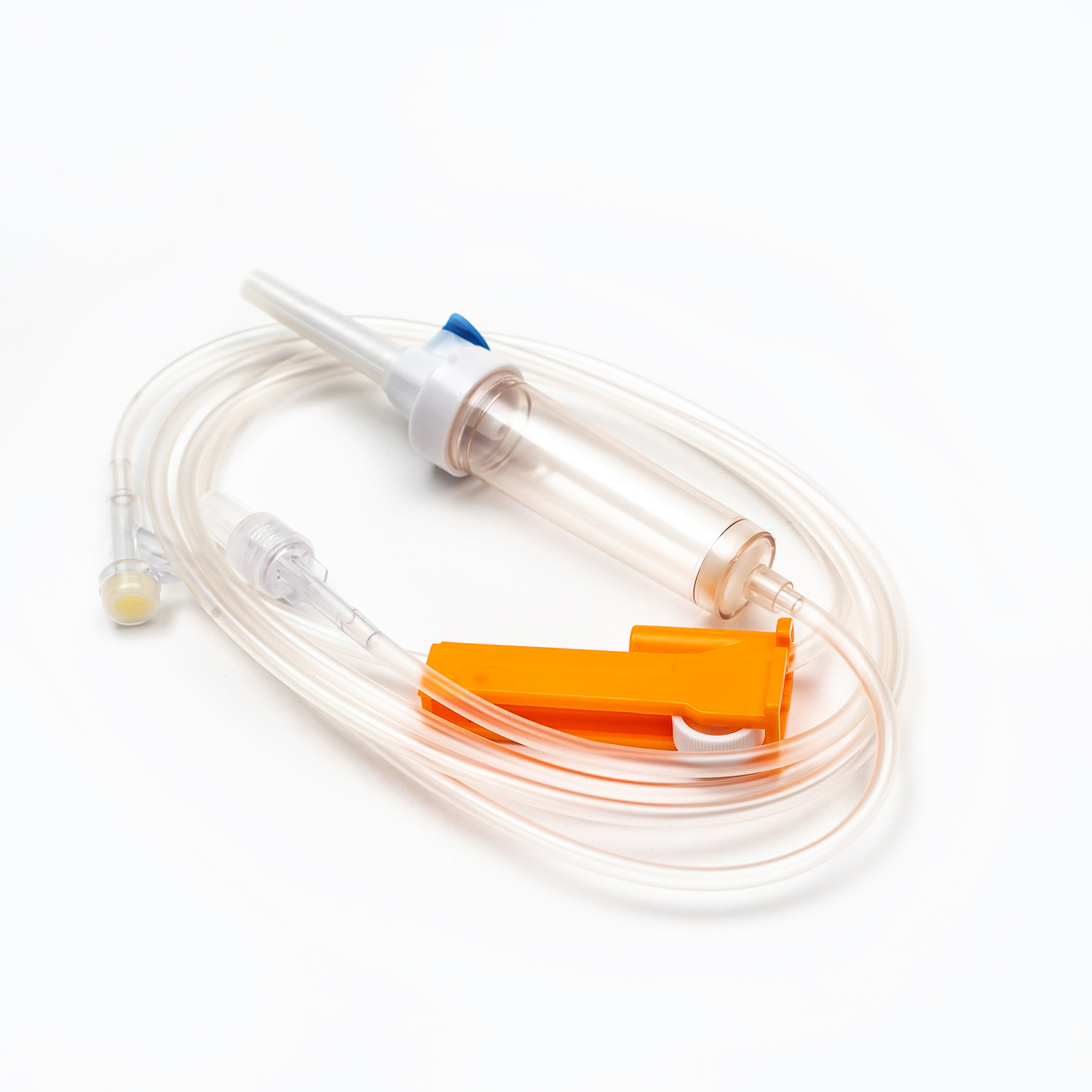
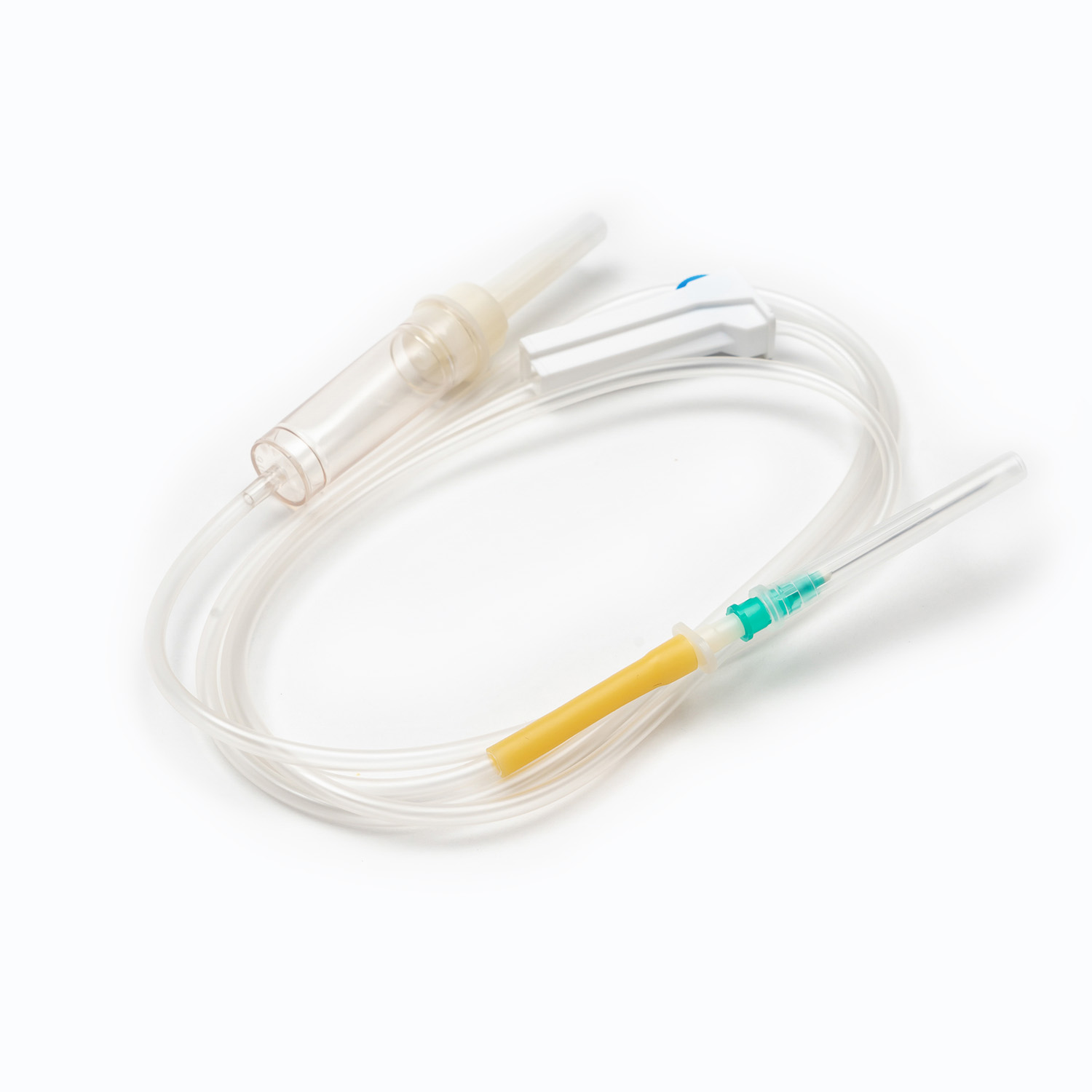
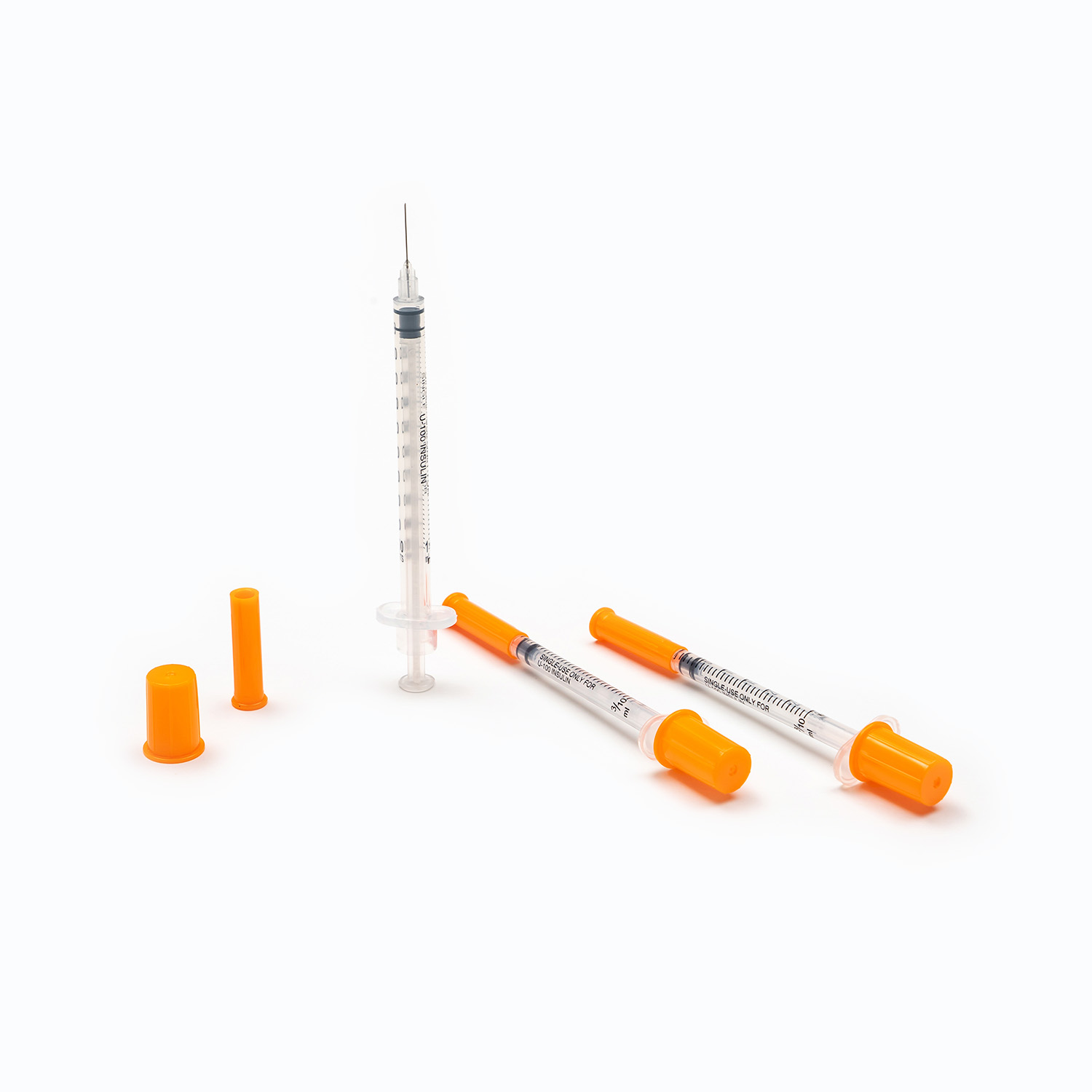
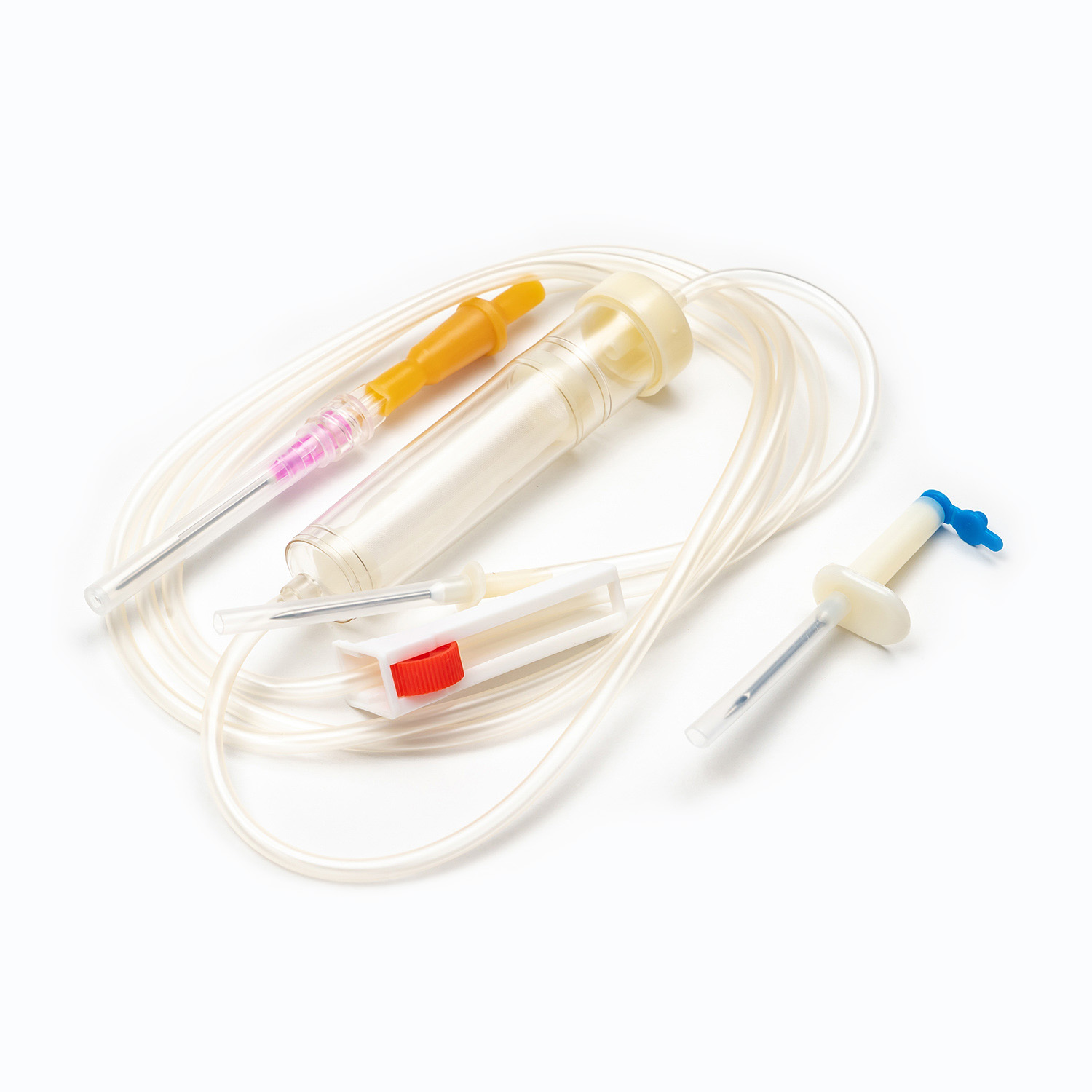
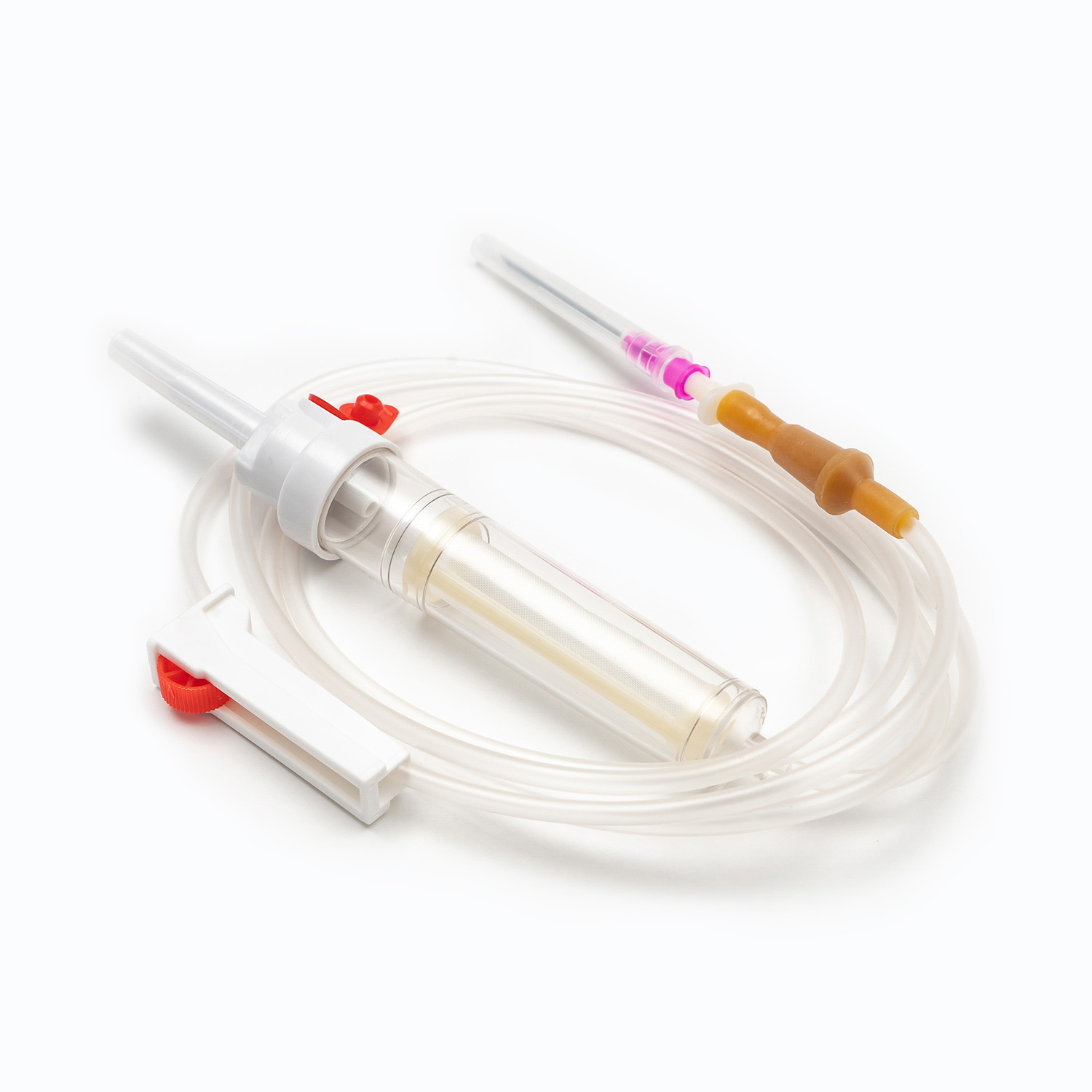
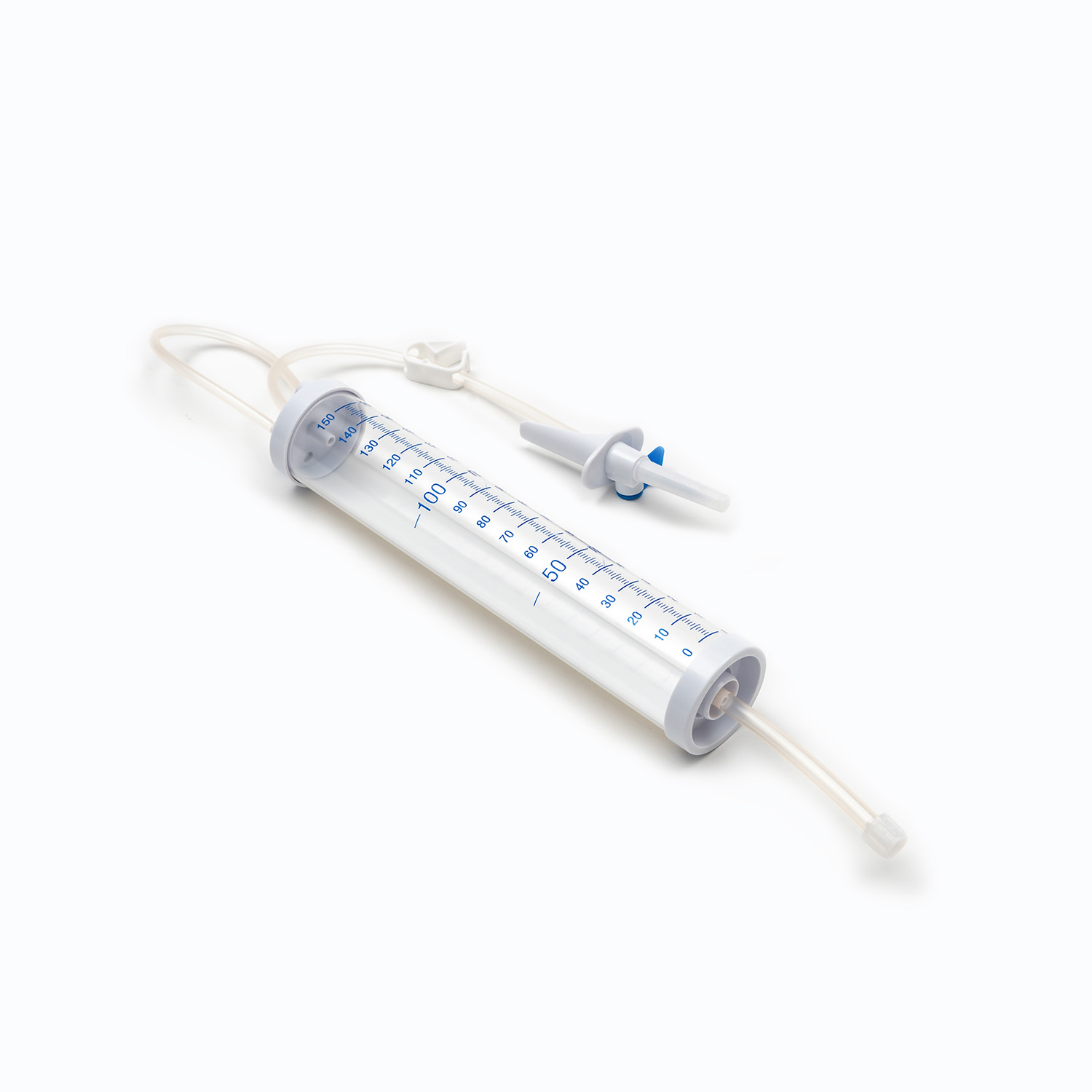
A disposable infusion set is a medical device used to deliver liquid medication or nutrient solutions from an infusion bottle or bag into the human venous system. It primarily consists of a needle, a drip chamber, a catheter, a flow regulator, an air filter, and an infusion needle (or infusion connector). Modern disposable infusion sets are typically made of medical-grade polymer materials, offering excellent transparency, strong flexibility, and stable pressure resistance, making it easier for medical personnel to monitor the flow rate and bubble status during infusion.
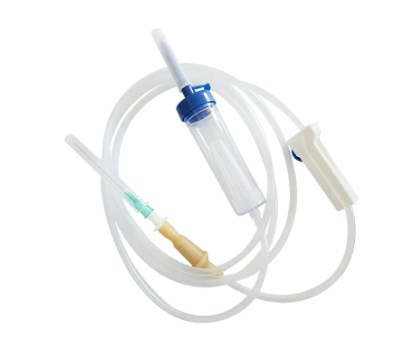
In terms of structural design, flow control and sealing performance are key indicators of an infusion set's quality. High-quality infusion sets should ensure a smooth, leak-free flow of medications and effectively prevent air from entering the vascular system, thereby reducing the risk of infusion complications. With the advancement of medical technology, some infusion sets have incorporated safety features such as automatic stop-flow, backflow prevention, and needlestick protection, further enhancing their convenience and safety in clinical use.
The Application of Polymer Materials in Disposable Infusion Sets
The key to disposable infusion sets lies in the choice of materials. Traditional infusion sets are mostly made of polyvinyl chloride (PVC). However, with increasing environmental and health requirements, medical non-PVC materials such as polyolefins (PO), polyethylene (PE), and polypropylene (PP) are becoming mainstream. These materials not only exhibit excellent biocompatibility and chemical stability, but also maintain excellent mechanical properties and transparency even after high-temperature sterilization.
Medical-grade materials must maintain their physical properties while resisting chemical reactions with the medication, preventing issues such as plasticizer precipitation and metal ion migration. Especially during the infusion of antibiotics, nutrient solutions, and chemotherapy drugs, the material's inertness and stability are directly related to the safety and effectiveness of the medication components. Disposable infusion sets made of non-PVC materials have gradually become the preferred choice of high-end medical institutions. This not only meets international environmental standards but also reflects the trend of the medical device industry towards safety, non-toxicity, and sustainability.
The Importance of Production Process and Quality Control
The manufacturing process of disposable infusion sets involves multiple steps, including injection molding, assembly, sealing, sterilization, and quality testing. Each step must strictly adhere to medical device production standards to ensure product sterility and consistency. Modern production lines are typically equipped with clean rooms and fully automated assembly equipment, ensuring that everything from raw material input to finished product packaging is completed in a dust-free environment to reduce the risk of human contamination.
In terms of quality testing, flow uniformity, sealing, particle detection, and needle sharpness testing are key control indicators in the industry. In particular, when testing fluid dynamics, manufacturers must ensure stable infusion flow rates with minimal error to ensure accurate drug delivery. Regulations on disposable infusion sets in the international medical market are becoming increasingly stringent, and compliance with ISO, CE, and FDA certification standards has become a fundamental requirement for companies to enter the global market.
The Clinical Application Value of Disposable Infusion Sets
Disposable infusion sets are used throughout every aspect of clinical treatment in hospitals, clinics, and home care settings. Their greatest value lies in ensuring aseptic operation and preventing cross-infection. Traditional reusable infusion sets are gradually being phased out, while disposable infusion sets, with their "use-and-disposable" nature, have significantly improved infusion safety standards.
Modern medical institutions are increasingly prioritizing the patient experience, and infusion set designs are evolving towards comfort and user-friendly features. For example, flexible tubing reduces discomfort during patient movement, precise flow regulators ensure smoother drug delivery, and transparent designs allow medical staff to monitor drug status in real time. These details reflect the deepening of the humanized design concept for medical devices and the overall improvement in the quality of medical services.
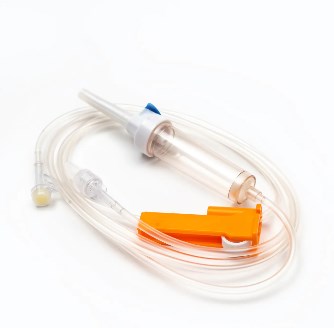
As an indispensable basic device in clinical practice, disposable infusion sets not only bear the responsibility for safe infusion but also represent the direction of medical device technological advancement. From material innovation to intelligent manufacturing, from safety design to environmental protection, each iteration of the infusion set reflects the medical industry's deep consideration and continuous pursuit of life and health. In the future, with the improvement of global medical standards and the enhancement of patient safety awareness, disposable infusion sets will play a more critical role in the medical system, providing more efficient and reliable guarantees for clinical treatment.



 English
English Français
Français русский
русский Español
Español